Copper »
PDB 8prq-8ryv »
8rfy »
Copper in PDB 8rfy: High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy]
Protein crystallography data
The structure of High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy], PDB code: 8rfy
was solved by
S.L.Rose,
F.M.Ferroni,
S.V.Antonyuk,
R.R.Eady,
S.S.Hasnain,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.00 / 1.79 |
| Space group | P 21 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 107.62, 107.62, 107.62, 90, 90, 90 |
| R / Rfree (%) | 17 / 21 |
Copper Binding Sites:
The binding sites of Copper atom in the High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy]
(pdb code 8rfy). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy], PDB code: 8rfy:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy], PDB code: 8rfy:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 8rfy
Go back to
Copper binding site 1 out
of 2 in the High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy]
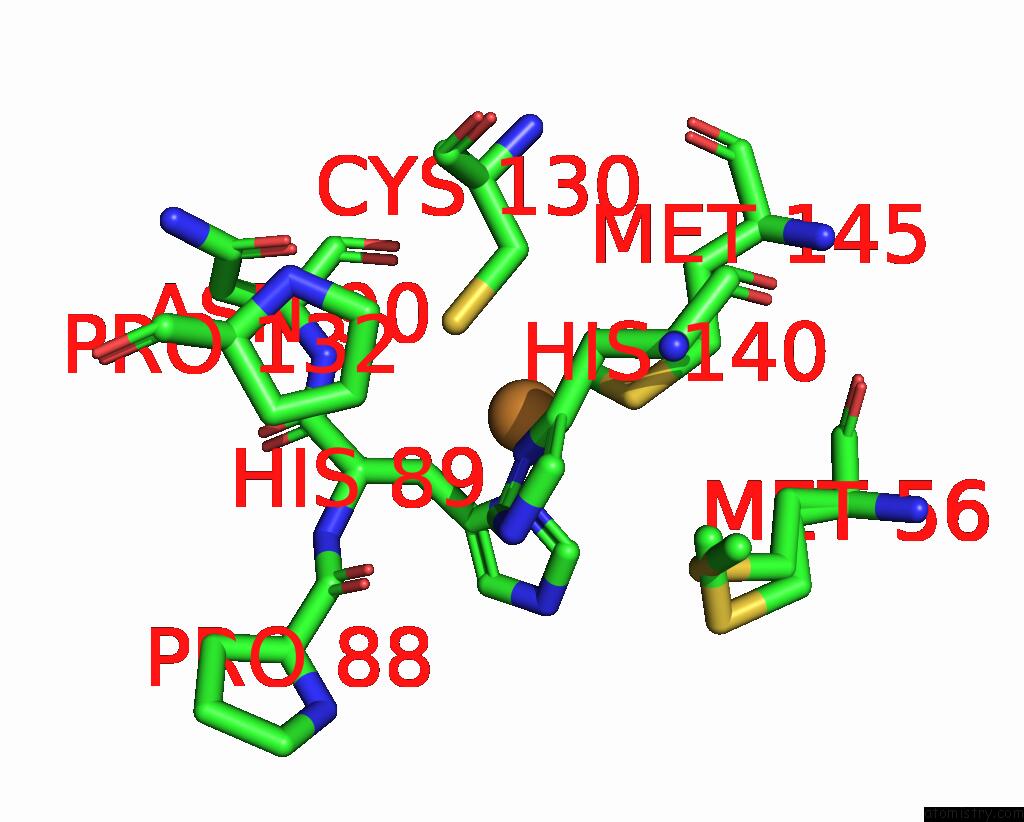
Mono view
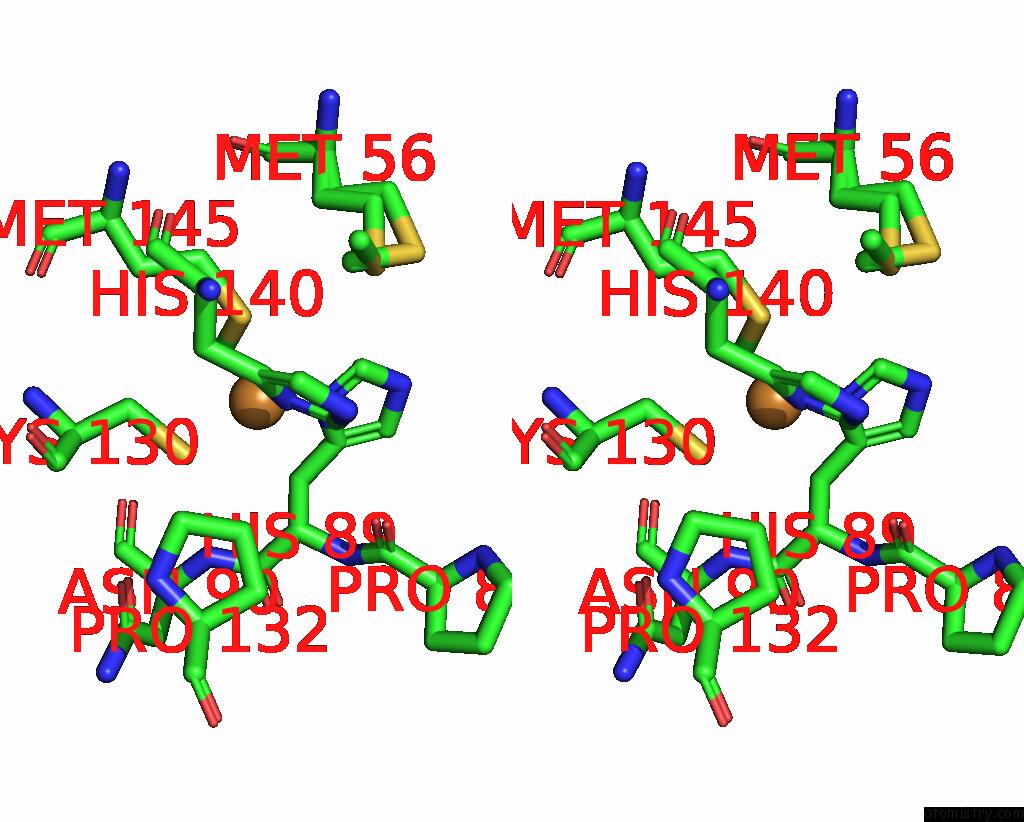
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy] within 5.0Å range:
|
Copper binding site 2 out of 2 in 8rfy
Go back to
Copper binding site 2 out
of 2 in the High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy]
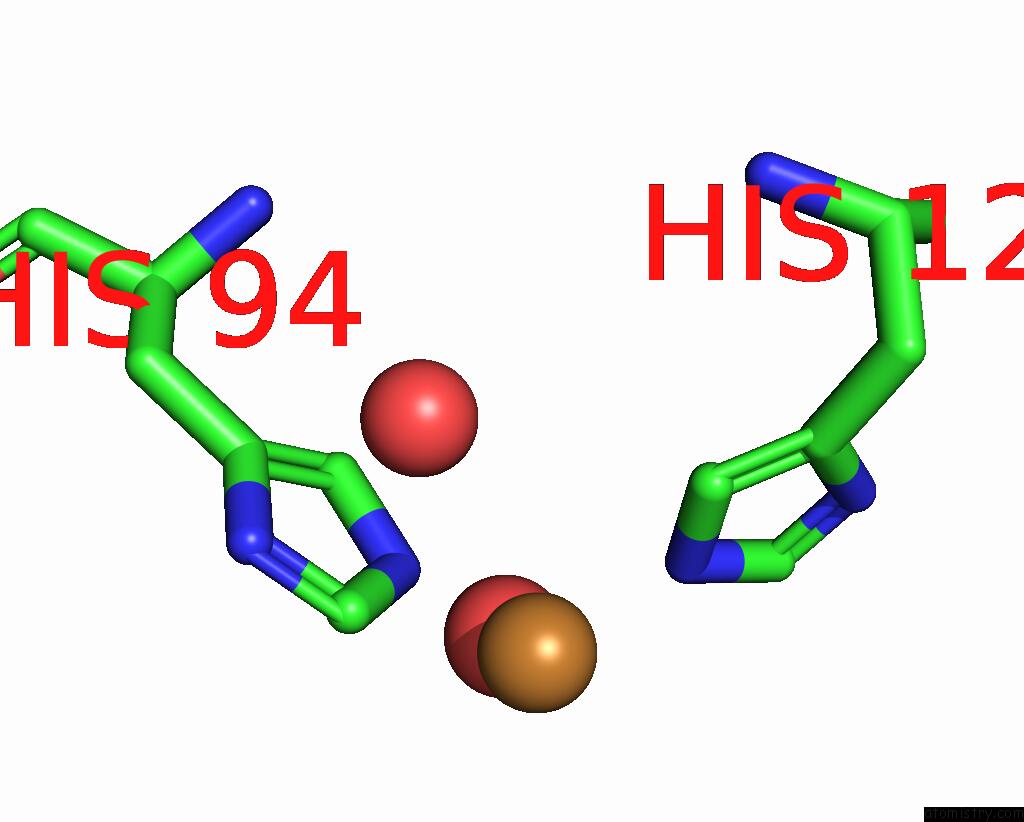
Mono view
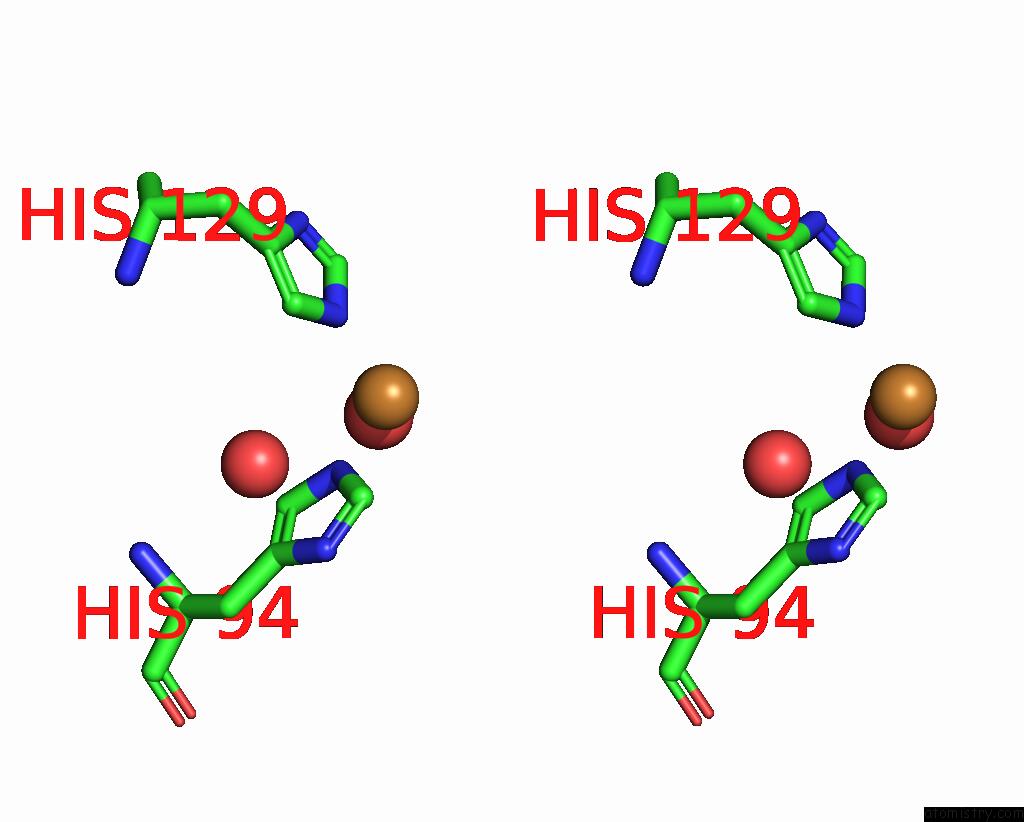
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of High pH (8.0) As-Isolated Msox Movie Series Dataset 30 of the Copper Nitrite Reductase From Bradyrhizobium Sp. ORS375 (Two-Domain)[20.40 Mgy] within 5.0Å range:
|
Reference:
S.L.Rose,
F.Martin Ferroni,
S.Horrell,
C.Dante Brondino,
R.R.Eady,
S.Jaho,
M.A.Hough,
R.L.Owen,
S.V.Antonyuk,
S.Samar Hasnain.
Spectroscopically Validated pH-Dependent Msox Movies Provide Detailed Mechanism of Copper Nitrite Reductases. J.Mol.Biol. 68706 2024.
ISSN: ESSN 1089-8638
PubMed: 39002715
DOI: 10.1016/J.JMB.2024.168706
Page generated: Mon Jul 14 09:22:40 2025
ISSN: ESSN 1089-8638
PubMed: 39002715
DOI: 10.1016/J.JMB.2024.168706
Last articles
I in 3SXVI in 3TNZ
I in 3S6L
I in 3T96
I in 3SV5
I in 3T3W
I in 3T3H
I in 3SIA
I in 3SSL
I in 3SSY