Copper »
PDB 8bae-8gbt »
8bpn »
Copper in PDB 8bpn: The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
Protein crystallography data
The structure of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus, PDB code: 8bpn
was solved by
L.A.Varfolomeeva,
A.Y.Solovieva,
N.S.Shipkov,
O.G.Kulikova,
N.I.Dergousova,
T.V.Rakitina,
K.M.Boyko,
T.V.Tikhonova,
V.O.Popov,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 81.06 / 1.99 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 90.559, 162.122, 90.861, 90, 119.8, 90 |
| R / Rfree (%) | 17.4 / 21.4 |
Copper Binding Sites:
The binding sites of Copper atom in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
(pdb code 8bpn). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 8 binding sites of Copper where determined in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus, PDB code: 8bpn:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Copper where determined in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus, PDB code: 8bpn:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Copper binding site 1 out of 8 in 8bpn
Go back to
Copper binding site 1 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
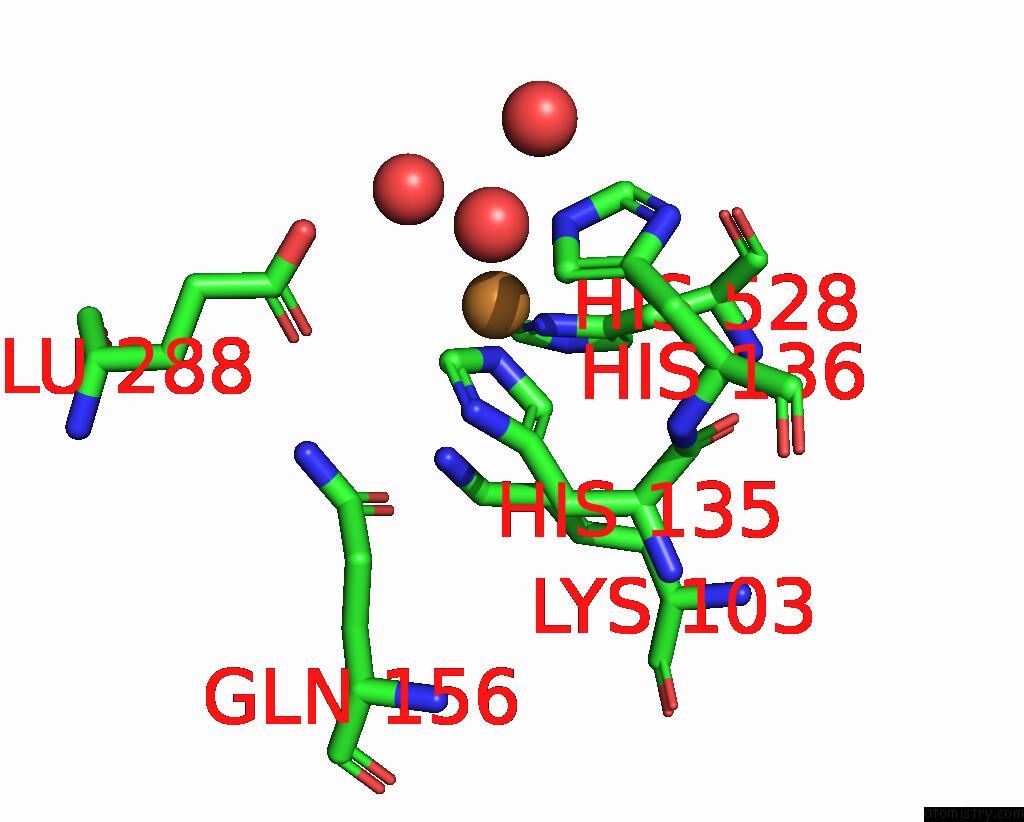
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
Copper binding site 2 out of 8 in 8bpn
Go back to
Copper binding site 2 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
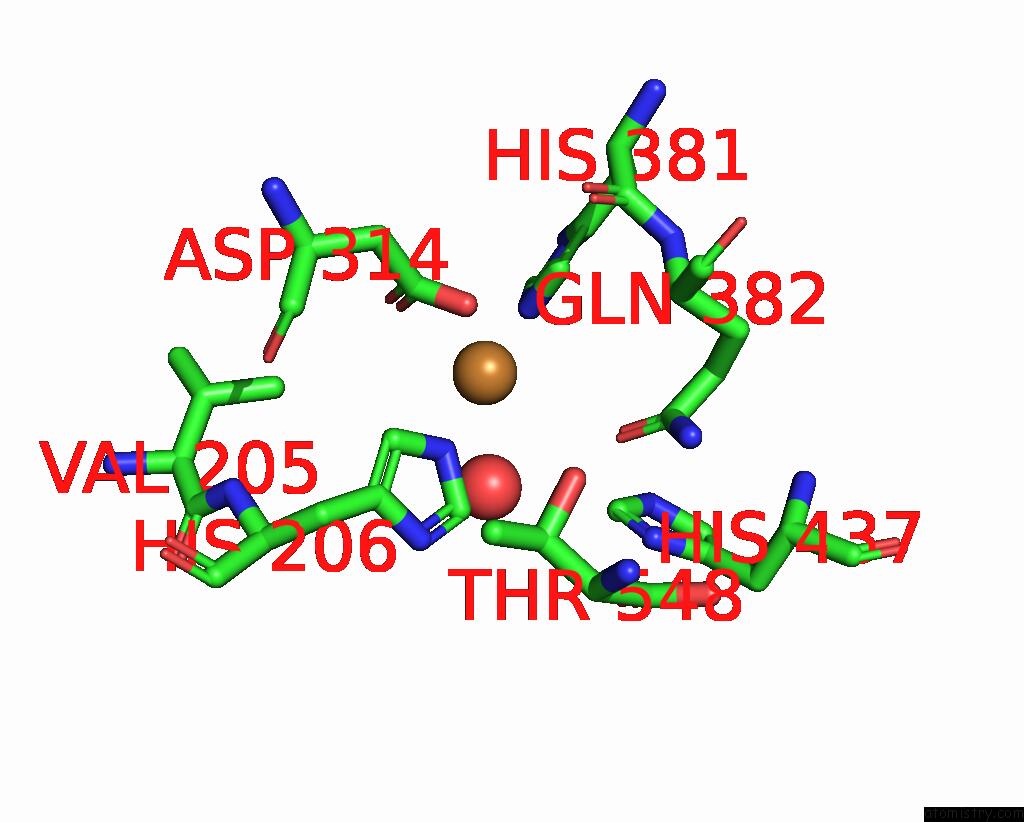
Mono view
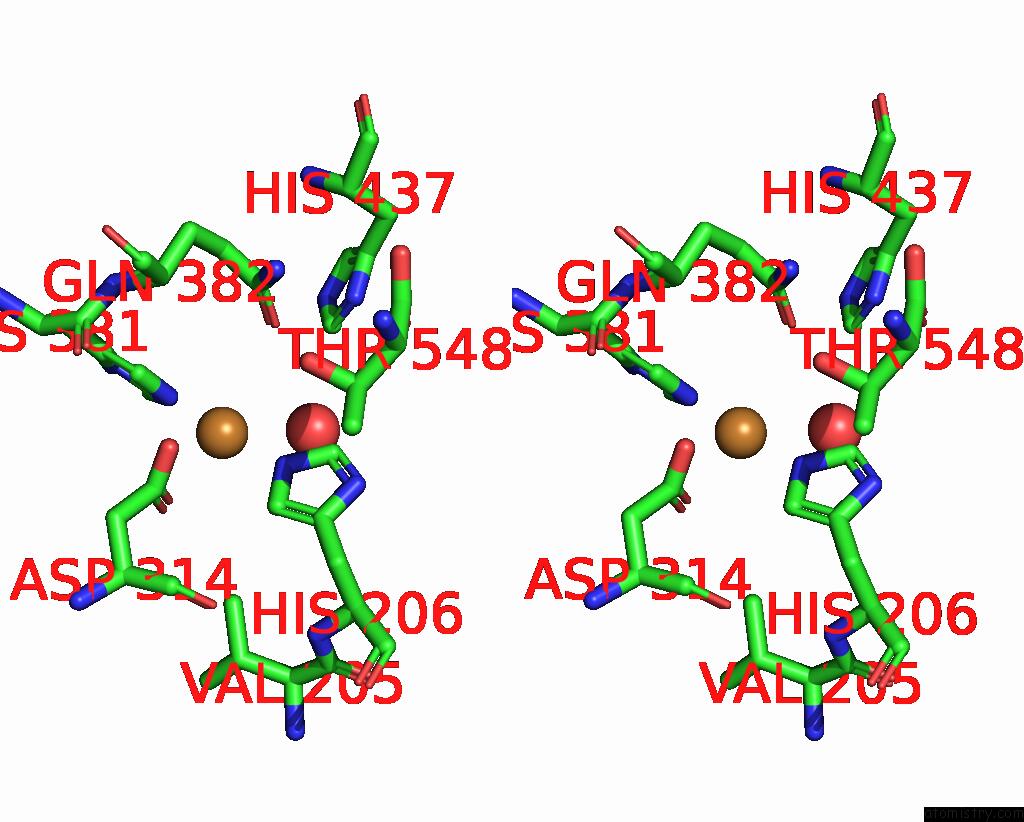
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
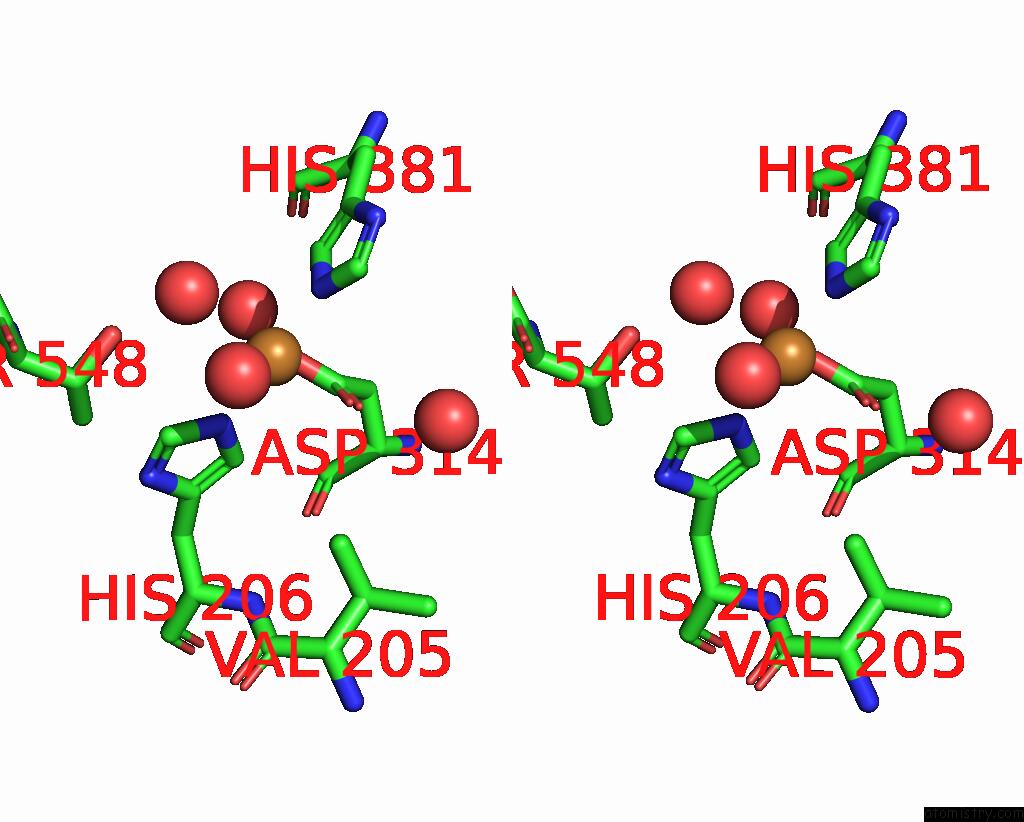
Copper binding site 3 out of 8 in 8bpn
Go back to
Copper binding site 3 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
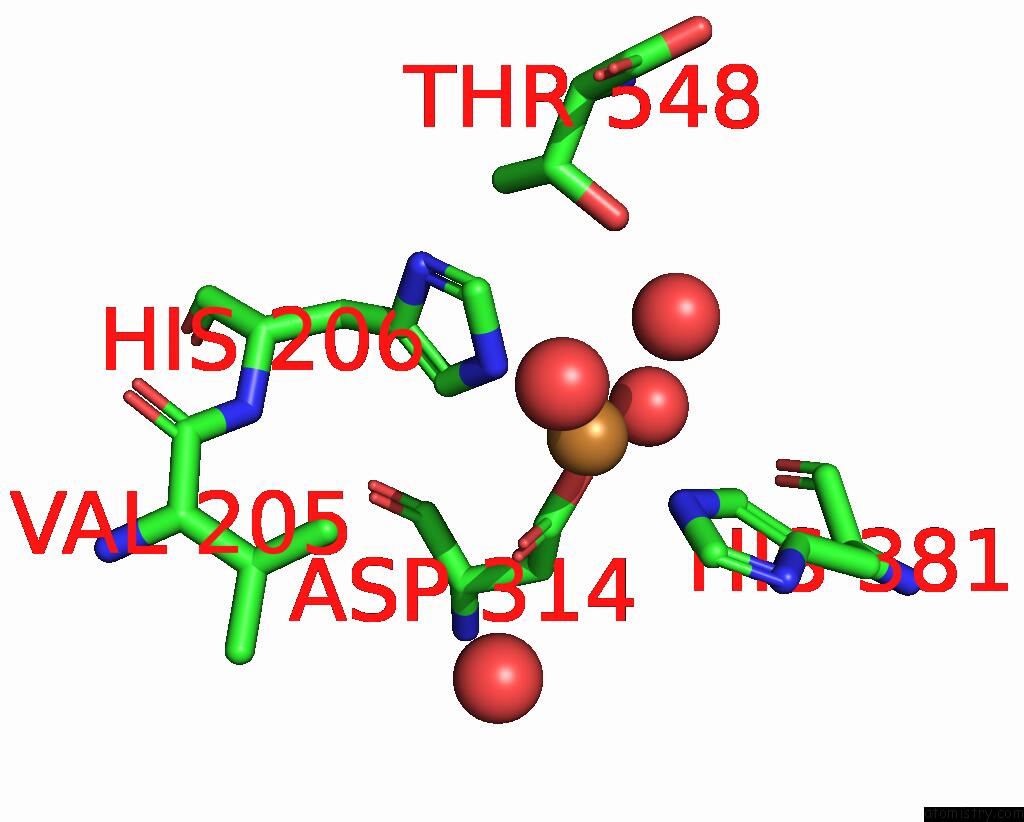
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
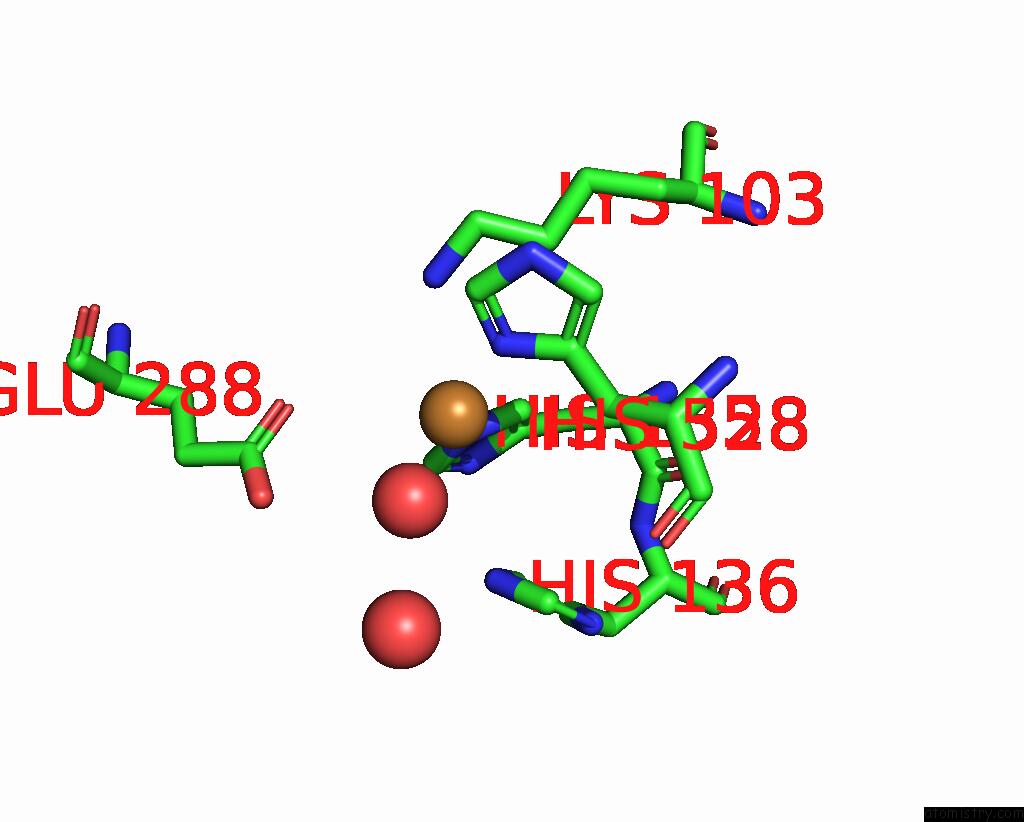
Copper binding site 4 out of 8 in 8bpn
Go back to
Copper binding site 4 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
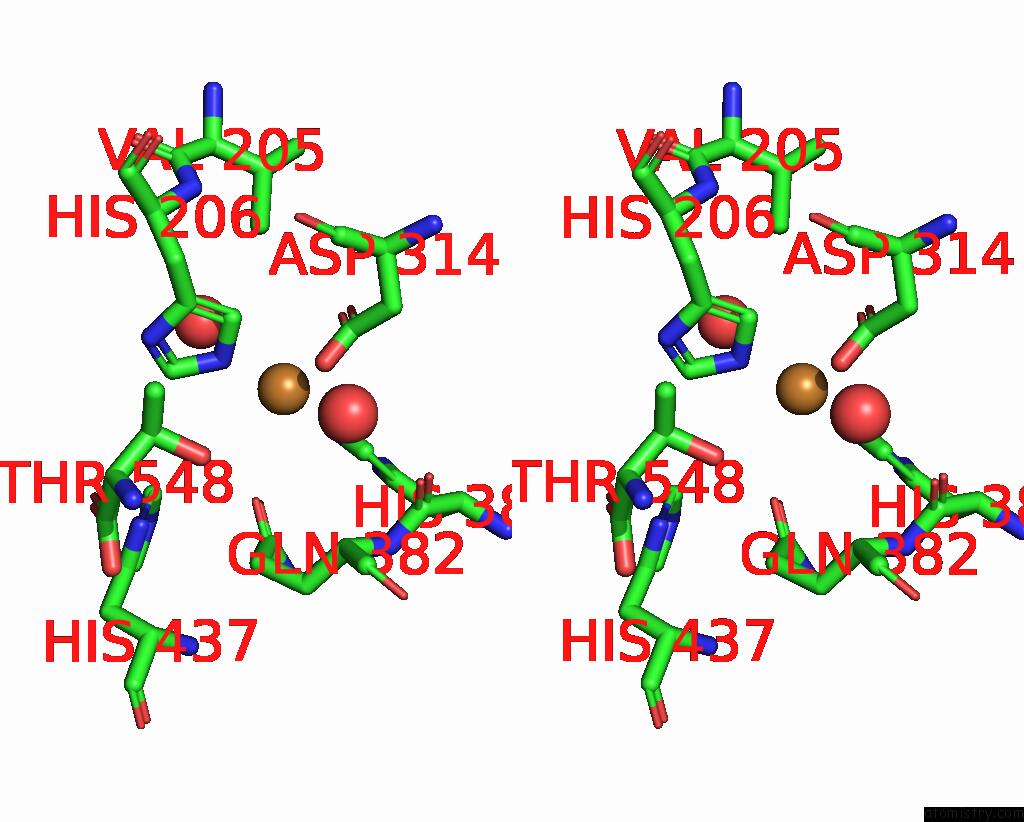
Copper binding site 5 out of 8 in 8bpn
Go back to
Copper binding site 5 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
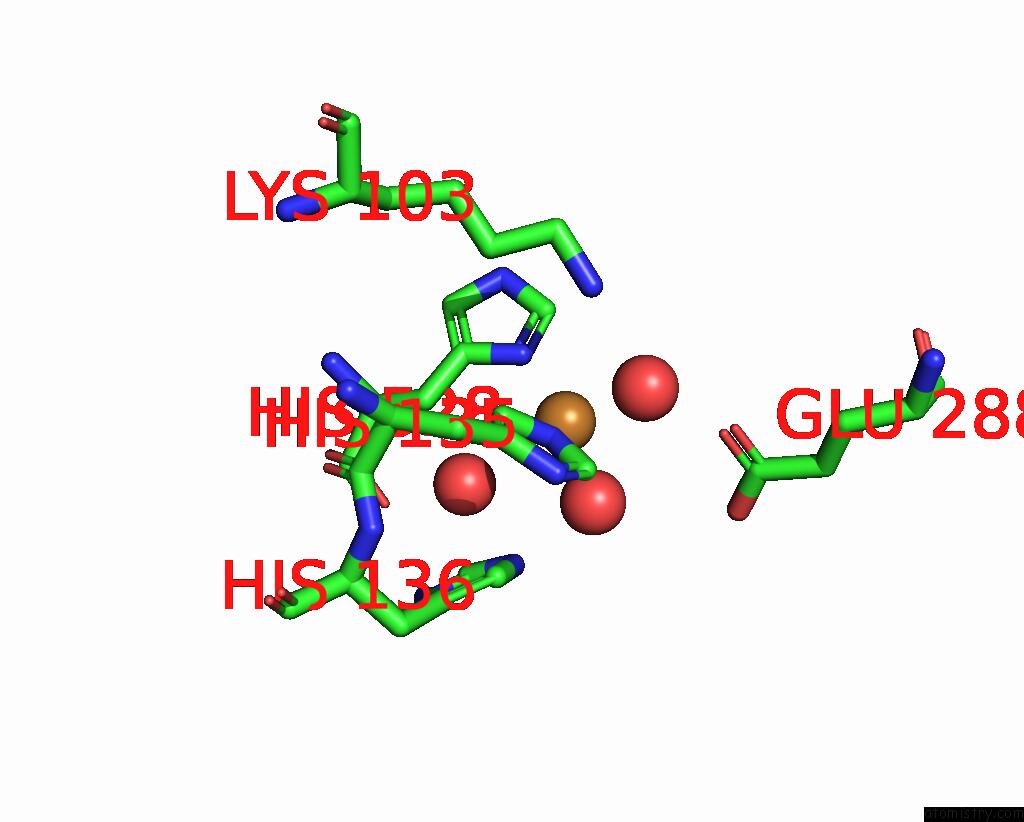
Copper binding site 6 out of 8 in 8bpn
Go back to
Copper binding site 6 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
Mono view
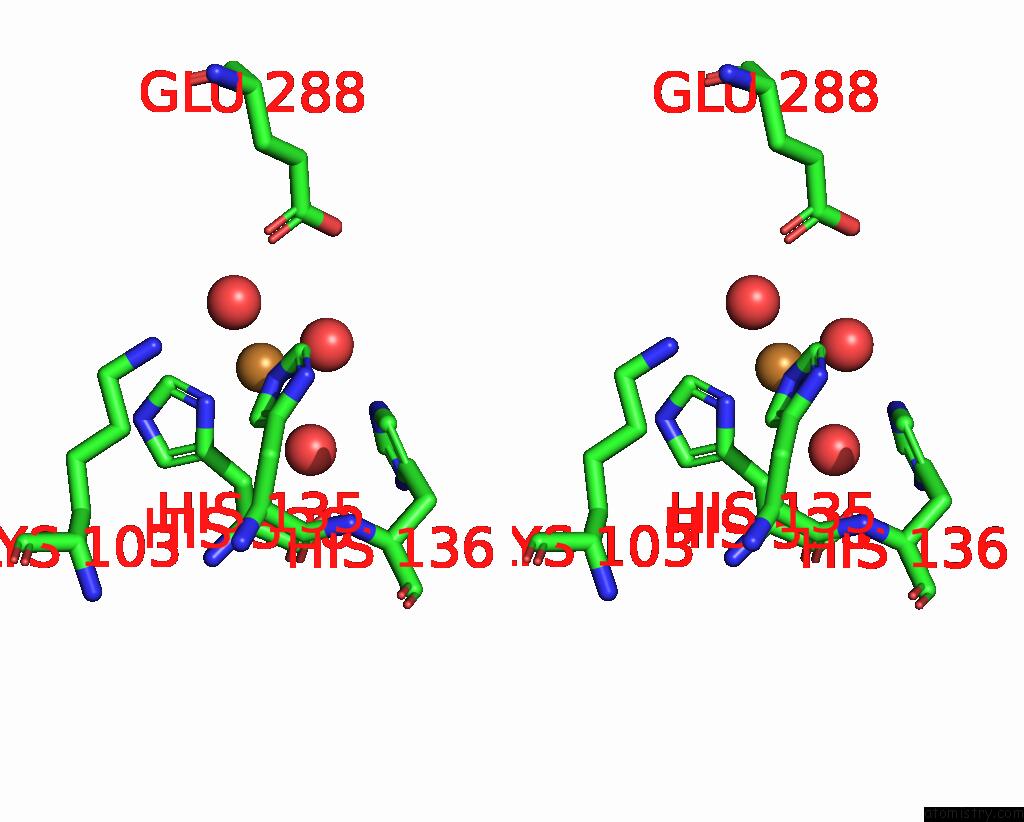
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
Copper binding site 7 out of 8 in 8bpn
Go back to
Copper binding site 7 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
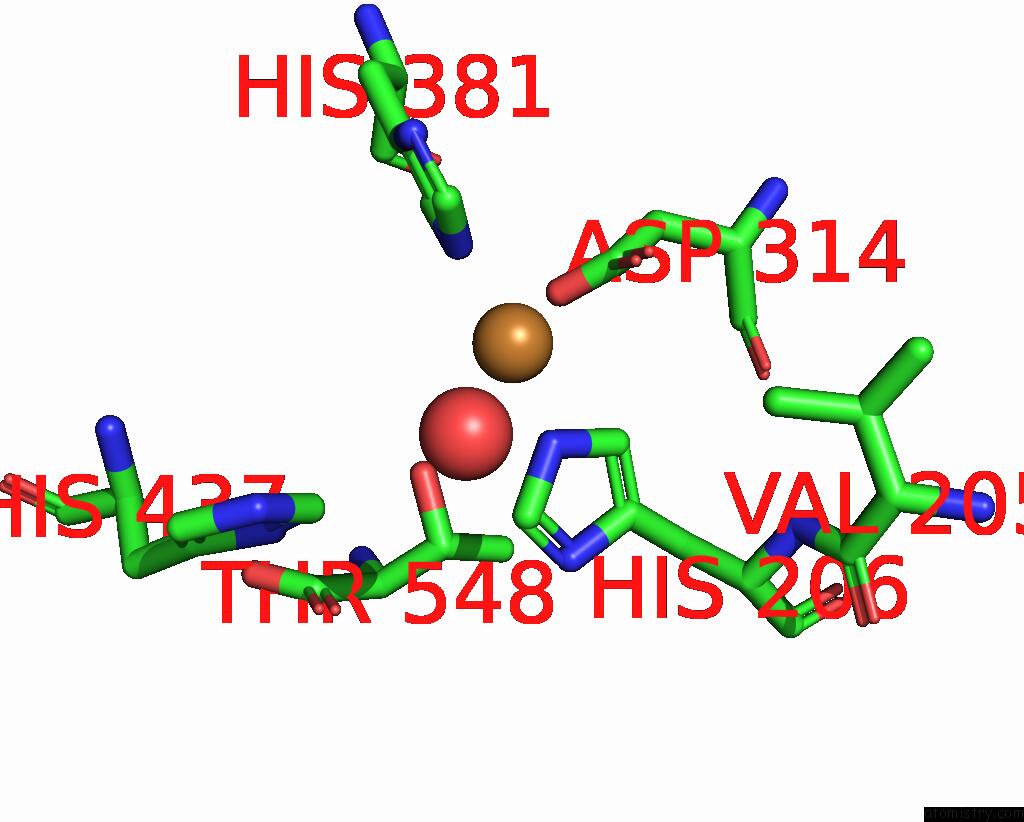
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 7 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
Copper binding site 8 out of 8 in 8bpn
Go back to
Copper binding site 8 out
of 8 in the The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus
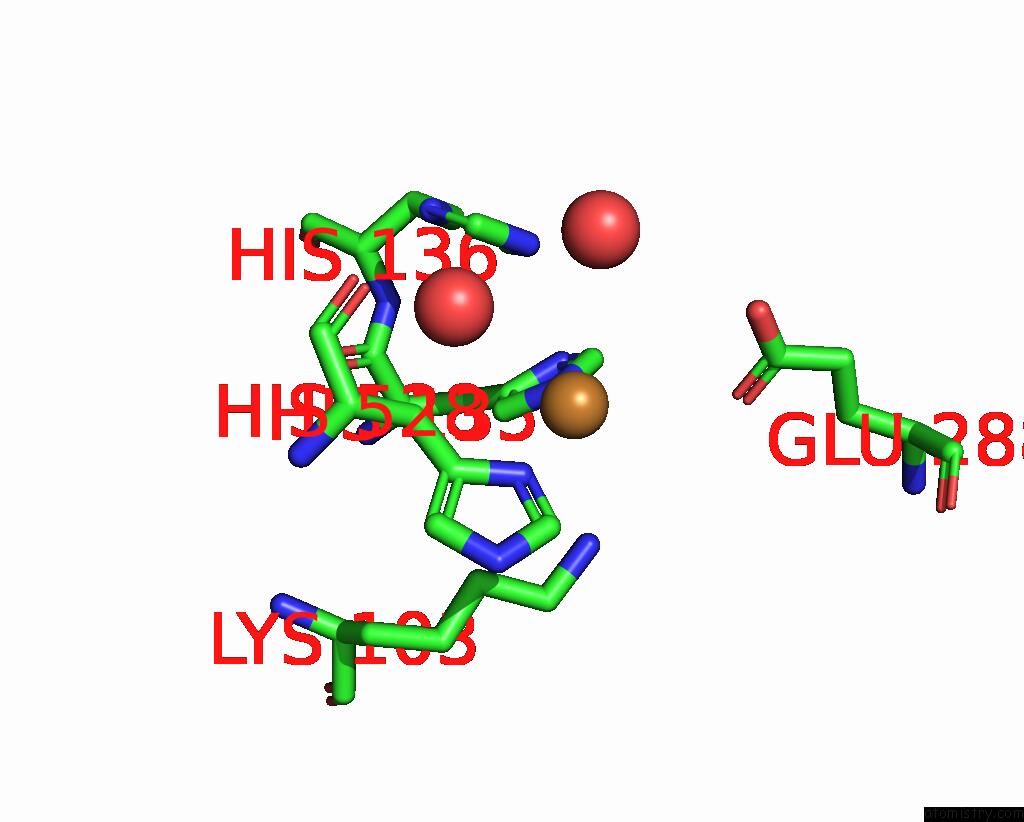
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 8 of The Structure of Thiocyanate Dehydrogenase Mutant Form with Phe 436 Replaced By Gln From Thioalkalivibrio Paradoxus within 5.0Å range:
|
Reference:
L.A.Varfolomeeva,
A.Y.Solovieva,
N.S.Shipkov,
O.G.Kulikova,
N.I.Dergousova,
T.V.Rakitina,
K.M.Boyko,
T.V.Tikhonova,
V.O.Popov.
Probing the Role of A Conserved Phenylalanine in the Active Site of Thiocyanate Dehydrogenase Crystals V. 12 2022.
ISSN: ESSN 2073-4352
DOI: 10.3390/CRYST12121787
Page generated: Mon Jul 14 08:48:53 2025
ISSN: ESSN 2073-4352
DOI: 10.3390/CRYST12121787
Last articles
Fe in 9GYRFe in 9GYZ
Fe in 9GYU
Fe in 9GYY
Fe in 9GMC
Fe in 9GM3
Fe in 9GYD
Fe in 9G7I
Fe in 9GTJ
Fe in 9GS2