Copper »
PDB 5ce9-5i0y »
5f7b »
Copper in PDB 5f7b: Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
Enzymatic activity of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
All present enzymatic activity of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K:
1.7.2.1;
1.7.2.1;
Protein crystallography data
The structure of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K, PDB code: 5f7b
was solved by
Y.Fukuda,
K.M.Tse,
T.Nakane,
T.Nakatsu,
M.Suzuki,
M.Sugahara,
S.Inoue,
T.Masuda,
F.Yumoto,
N.Matsugaki,
E.Nango,
K.Tono,
Y.Joti,
T.Kameshima,
C.Song,
T.Hatsui,
M.Yabashi,
O.Nureki,
M.E.P.Murphy,
T.Inoue,
S.Iwata,
E.Mizohata,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.83 / 1.56 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.836, 103.511, 147.246, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.9 / 17.7 |
Copper Binding Sites:
The binding sites of Copper atom in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
(pdb code 5f7b). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 6 binding sites of Copper where determined in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K, PDB code: 5f7b:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Copper where determined in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K, PDB code: 5f7b:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
Copper binding site 1 out of 6 in 5f7b
Go back to
Copper binding site 1 out
of 6 in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
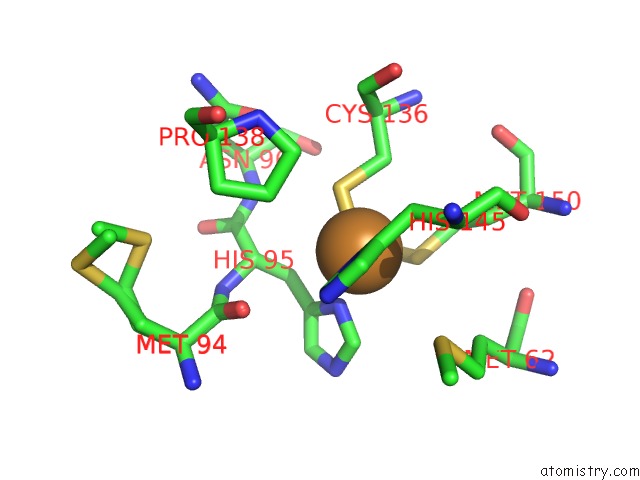
Mono view
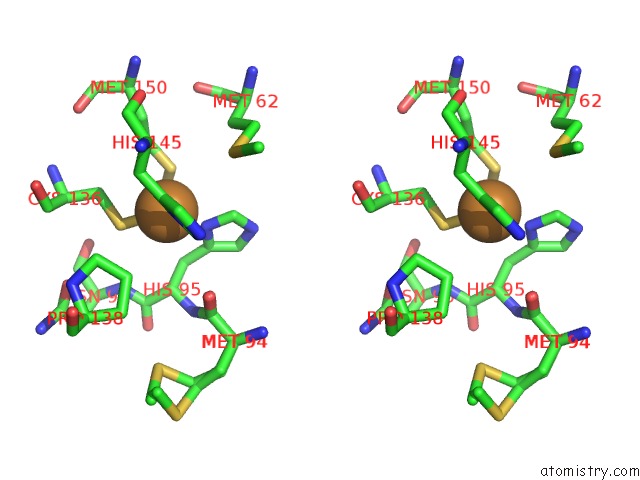
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K within 5.0Å range:
|
Copper binding site 2 out of 6 in 5f7b
Go back to
Copper binding site 2 out
of 6 in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
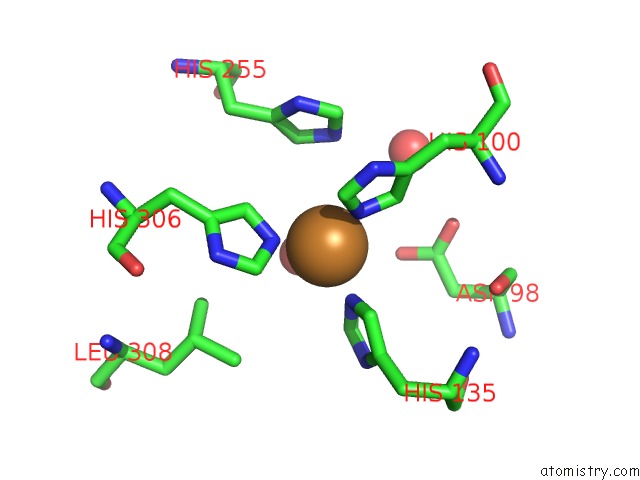
Mono view
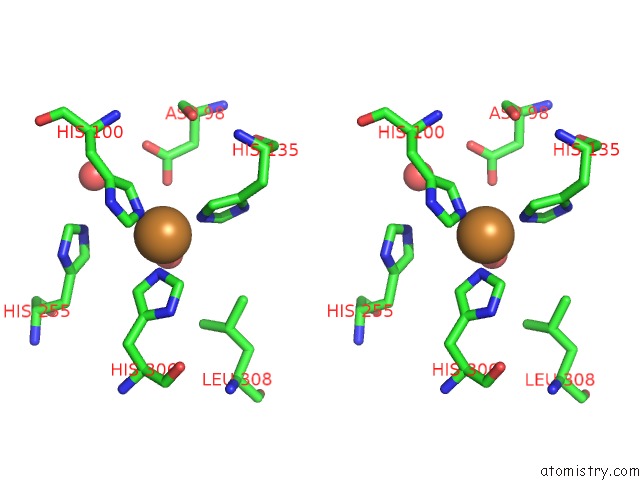
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K within 5.0Å range:
|
Copper binding site 3 out of 6 in 5f7b
Go back to
Copper binding site 3 out
of 6 in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
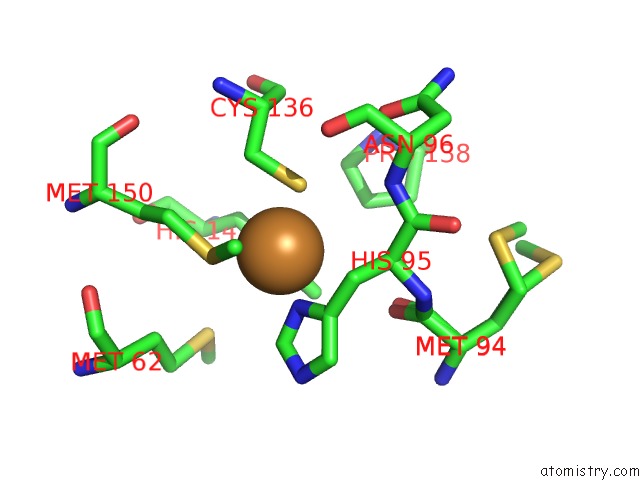
Mono view
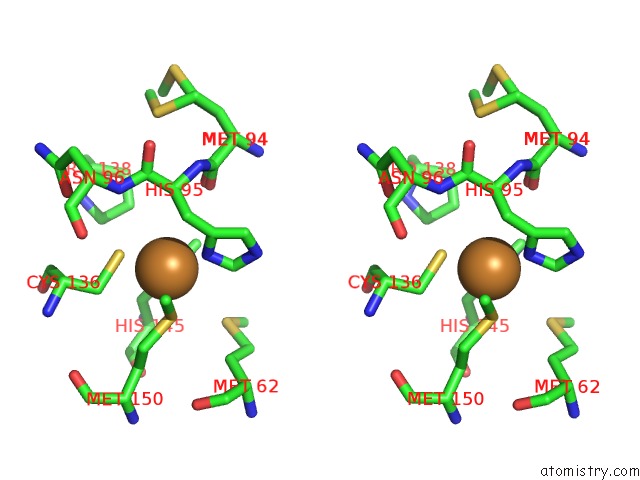
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K within 5.0Å range:
|
Copper binding site 4 out of 6 in 5f7b
Go back to
Copper binding site 4 out
of 6 in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
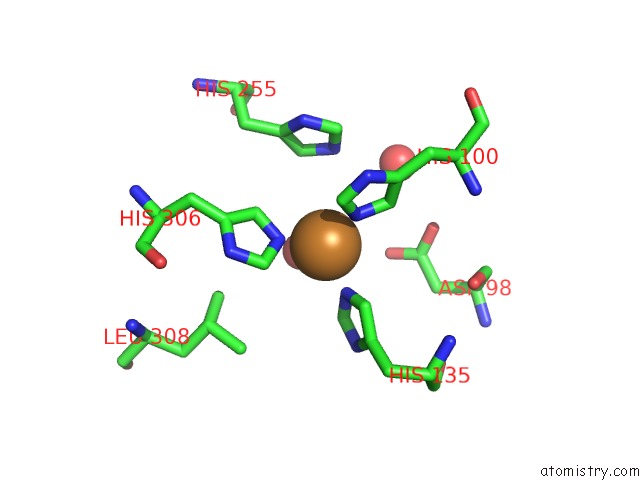
Mono view
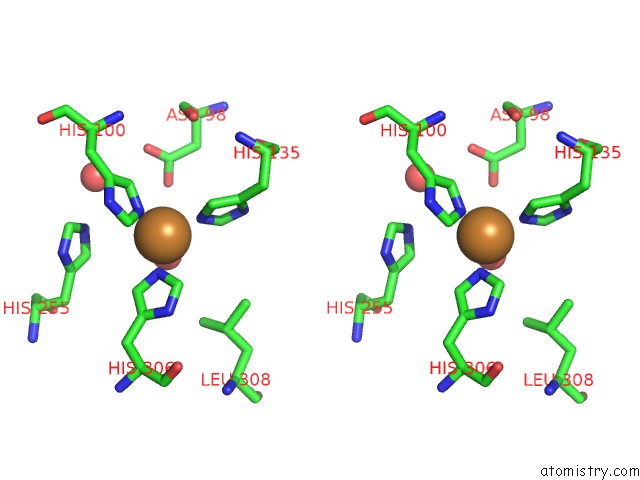
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K within 5.0Å range:
|
Copper binding site 5 out of 6 in 5f7b
Go back to
Copper binding site 5 out
of 6 in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
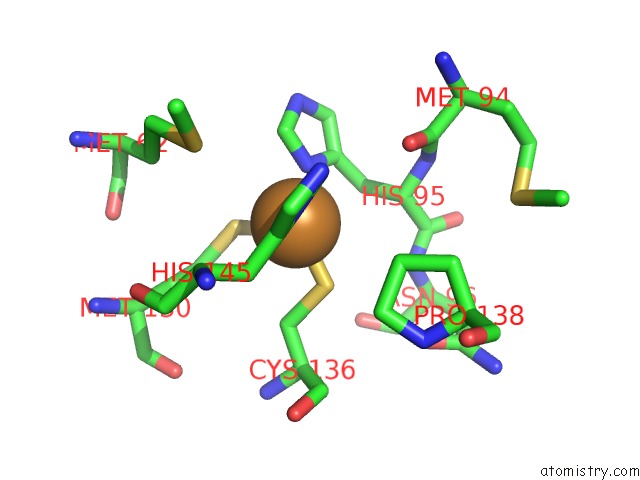
Mono view
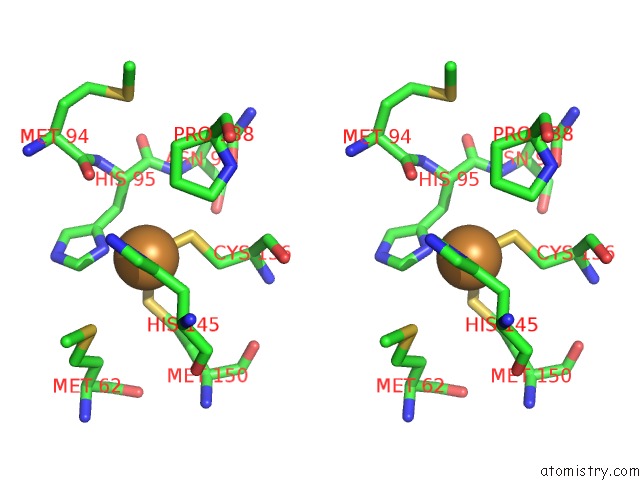
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K within 5.0Å range:
|
Copper binding site 6 out of 6 in 5f7b
Go back to
Copper binding site 6 out
of 6 in the Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K
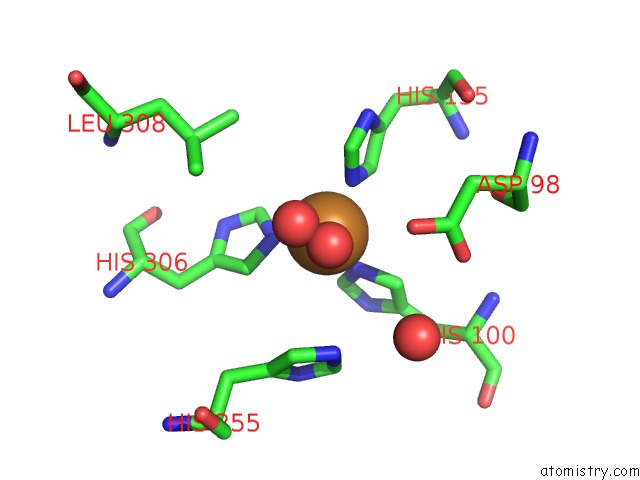
Mono view
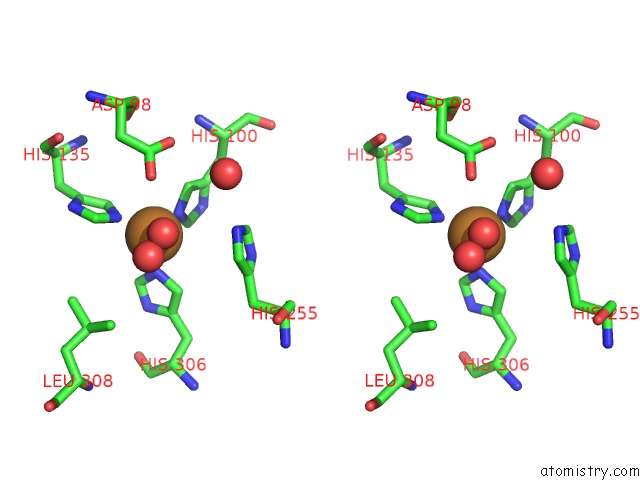
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Resting State Structure of Cunir Form Alcaligenes Faecalis Determined at 293 K within 5.0Å range:
|
Reference:
Y.Fukuda,
K.M.Tse,
T.Nakane,
T.Nakatsu,
M.Suzuki,
M.Sugahara,
S.Inoue,
T.Masuda,
F.Yumoto,
N.Matsugaki,
E.Nango,
K.Tono,
Y.Joti,
T.Kameshima,
C.Song,
T.Hatsui,
M.Yabashi,
O.Nureki,
M.E.P.Murphy,
T.Inoue,
S.Iwata,
E.Mizohata.
Redox-Coupled Proton Transfer Mechanism in Nitrite Reductase Revealed By Femtosecond Crystallography Proc.Natl.Acad.Sci.Usa V. 113 2928 2016.
ISSN: ESSN 1091-6490
PubMed: 26929369
DOI: 10.1073/PNAS.1517770113
Page generated: Mon Jul 14 04:33:29 2025
ISSN: ESSN 1091-6490
PubMed: 26929369
DOI: 10.1073/PNAS.1517770113
Last articles
I in 1HDYI in 1HC0
I in 1HC9
I in 1GWG
I in 1GZA
I in 1GWD
I in 1GUL
I in 1GTE
I in 1GTH
I in 1GJD