Copper »
PDB 7s1f-7xmb »
7t5e »
Copper in PDB 7t5e: Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange
Protein crystallography data
The structure of Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange, PDB code: 7t5e
was solved by
G.C.Schroder,
F.Meilleur,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | N/A / 1.90 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 68.296, 42.269, 70.413, 90, 98.47, 90 |
| R / Rfree (%) | 14.4 / 21.4 |
Copper Binding Sites:
The binding sites of Copper atom in the Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange
(pdb code 7t5e). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange, PDB code: 7t5e:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange, PDB code: 7t5e:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 7t5e
Go back to
Copper binding site 1 out
of 2 in the Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange

Mono view
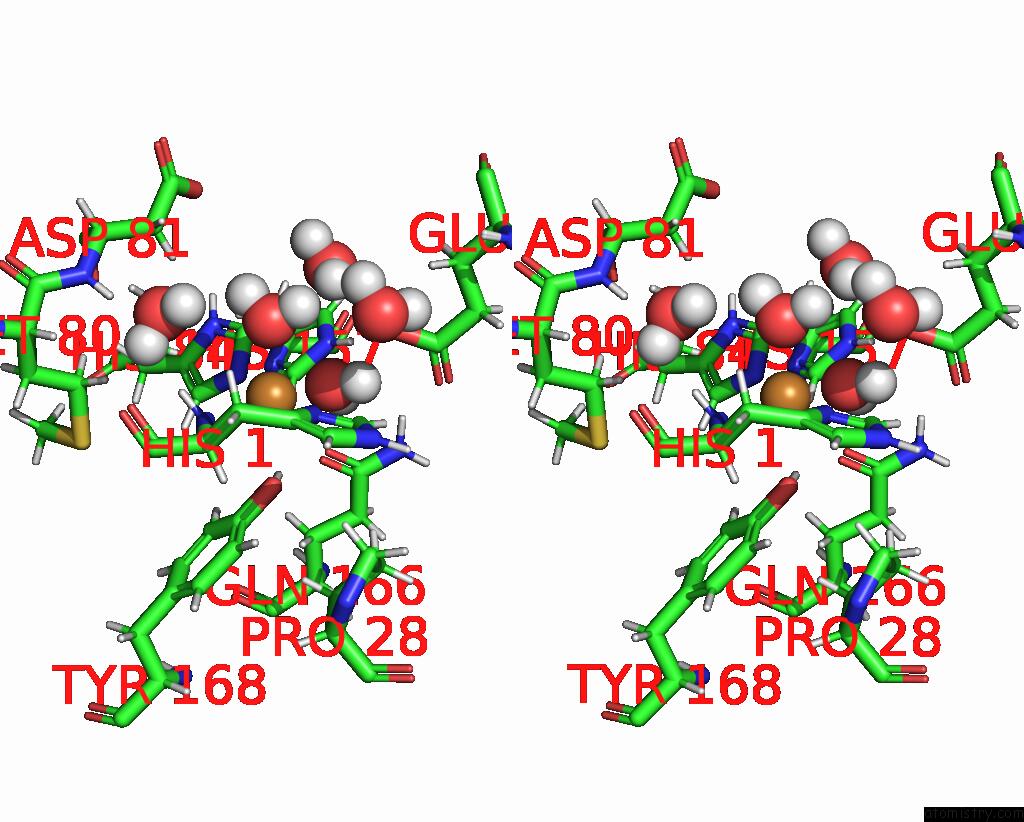
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange within 5.0Å range:
|
Copper binding site 2 out of 2 in 7t5e
Go back to
Copper binding site 2 out
of 2 in the Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Neutron Structure of Neurospora Crassa Polysaccharide Monooxygenase 9D (NCLPMO9D) Low pH Vapor Exchange within 5.0Å range:
|
Reference:
G.C.Schroder,
W.B.O'dell,
S.P.Webb,
P.K.Agarwal,
F.Meilleur.
Capture of Activated Dioxygen Intermediates at the Copper-Active Site of A Lytic Polysaccharide Monooxygenase. Chem Sci V. 13 13303 2022.
ISSN: ISSN 2041-6520
PubMed: 36507176
DOI: 10.1039/D2SC05031E
Page generated: Wed Jul 31 09:13:31 2024
ISSN: ISSN 2041-6520
PubMed: 36507176
DOI: 10.1039/D2SC05031E
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW