Copper »
PDB 7a8v-7ev7 »
7bdn »
Copper in PDB 7bdn: Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
Protein crystallography data
The structure of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form, PDB code: 7bdn
was solved by
K.Zovo,
S.Majumdar,
T.Lukk,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.50 / 2.70 |
| Space group | P 21 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 176.981, 176.981, 176.981, 90, 90, 90 |
| R / Rfree (%) | 20 / 22.2 |
Copper Binding Sites:
The binding sites of Copper atom in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
(pdb code 7bdn). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 6 binding sites of Copper where determined in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form, PDB code: 7bdn:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Copper where determined in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form, PDB code: 7bdn:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
Copper binding site 1 out of 6 in 7bdn
Go back to
Copper binding site 1 out
of 6 in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
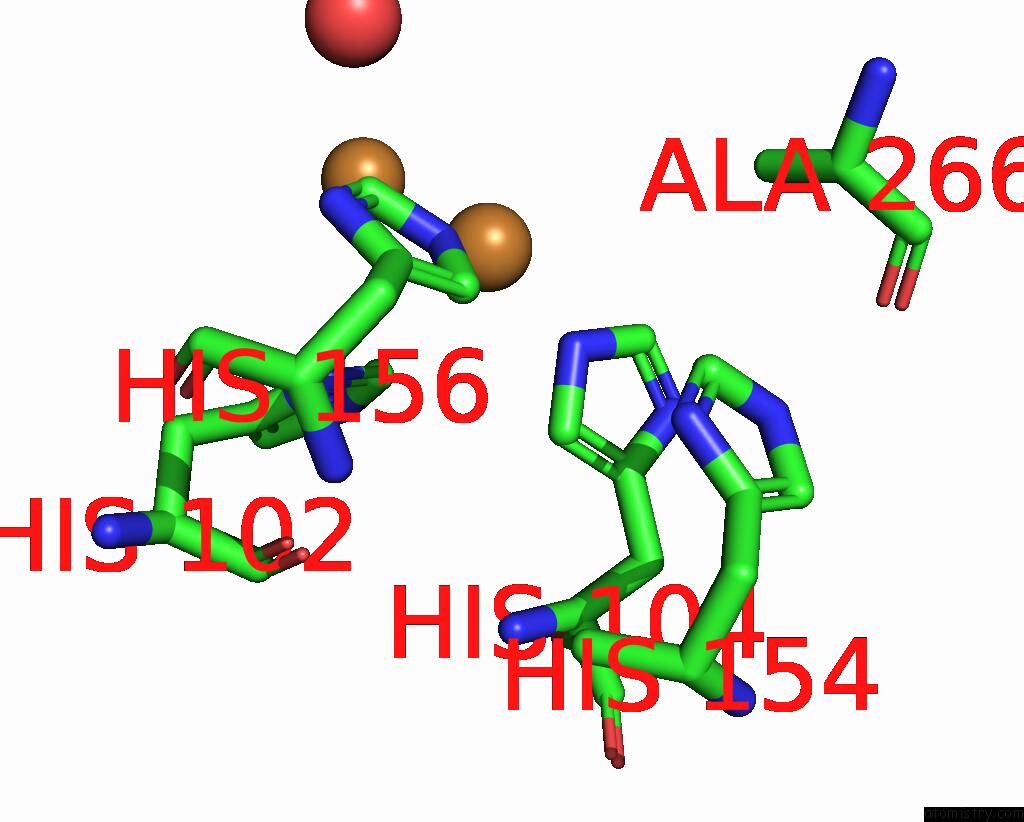
Mono view
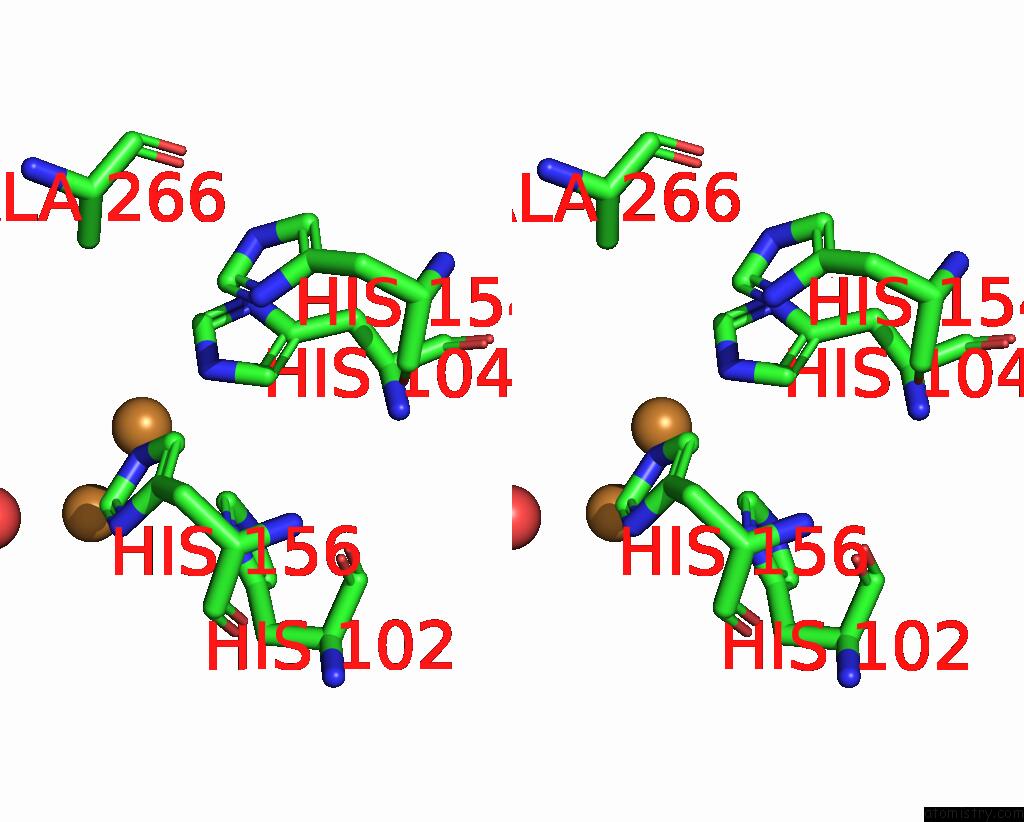
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form within 5.0Å range:
|
Copper binding site 2 out of 6 in 7bdn
Go back to
Copper binding site 2 out
of 6 in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
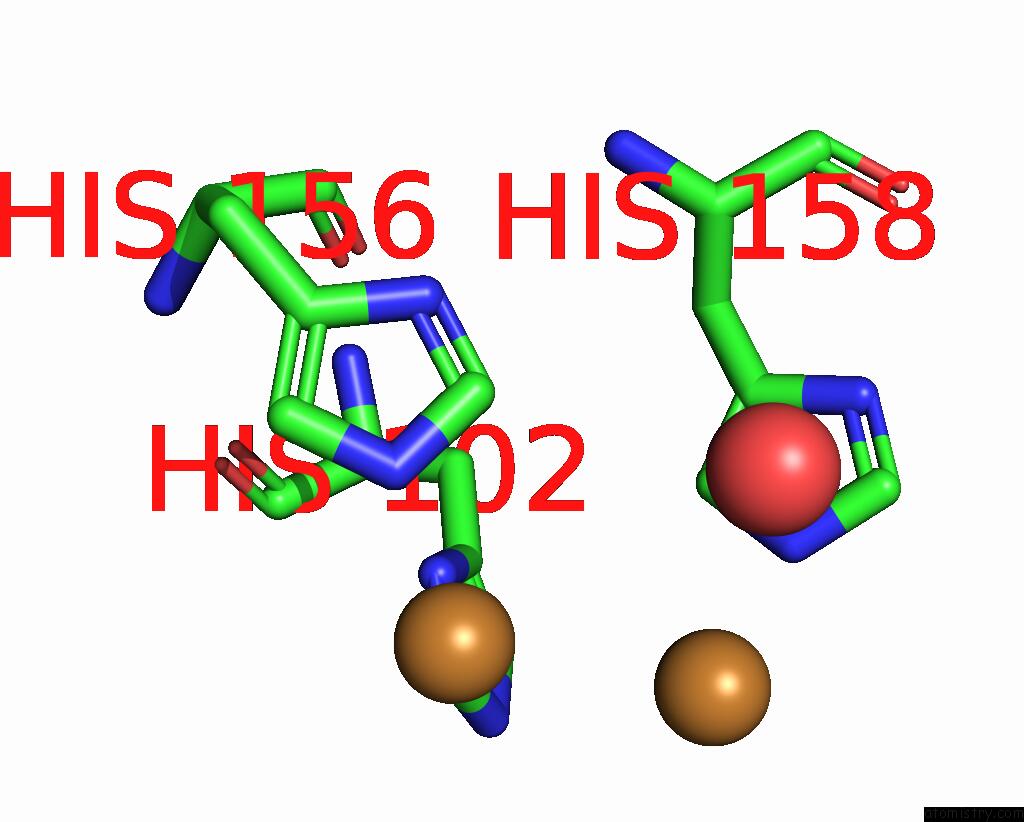
Mono view
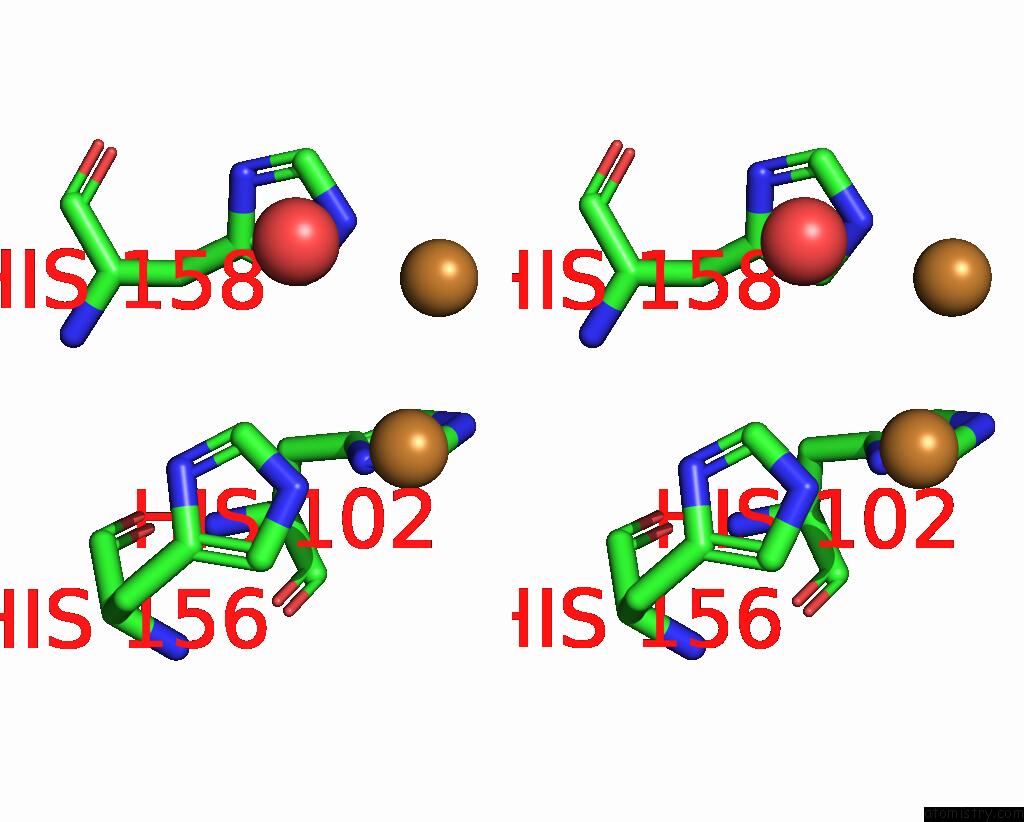
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form within 5.0Å range:
|
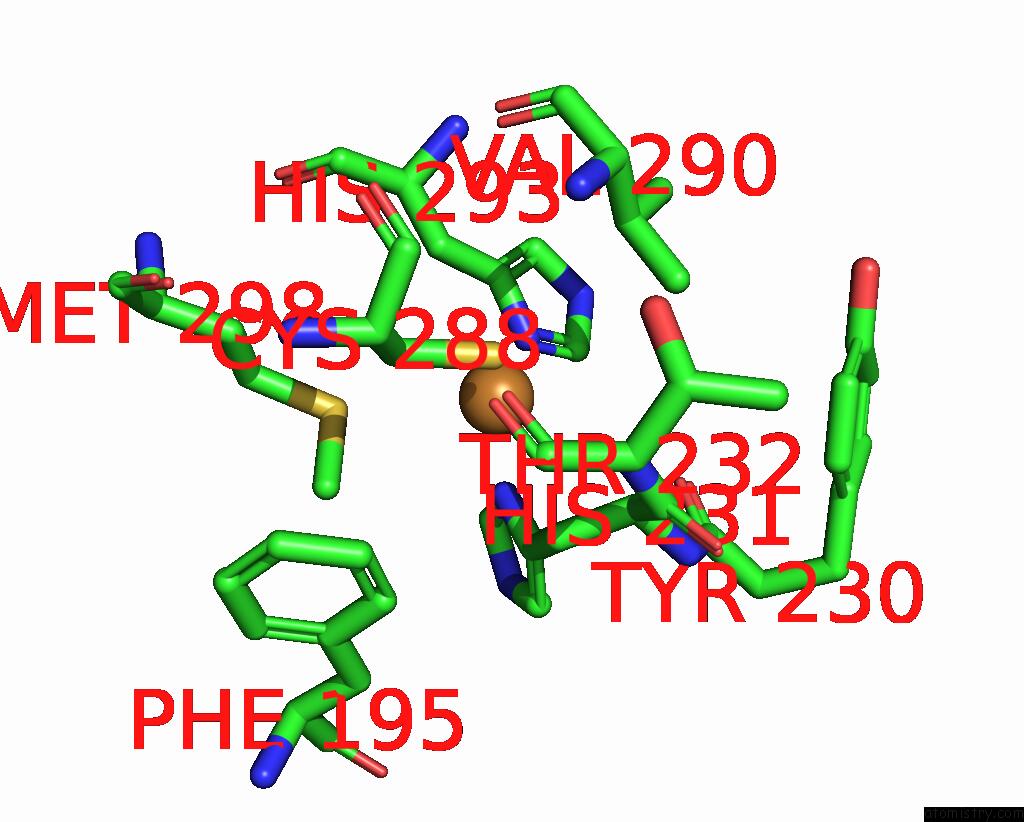
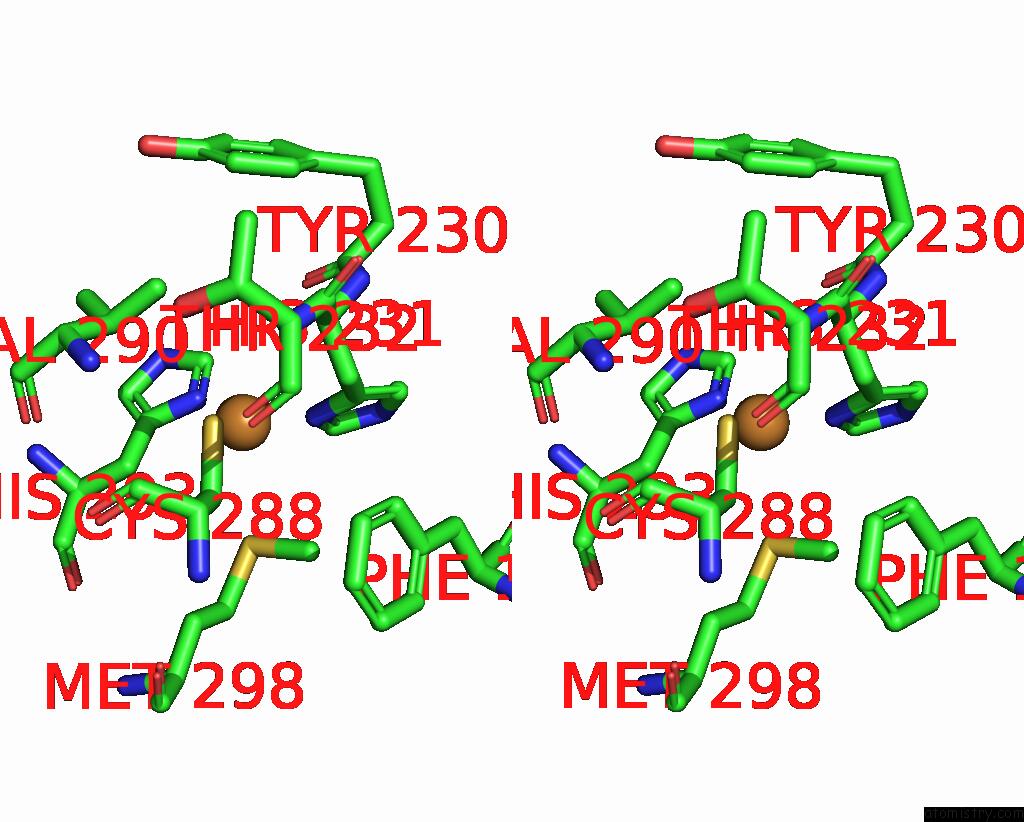
Copper binding site 3 out of 6 in 7bdn
Go back to
Copper binding site 3 out
of 6 in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form within 5.0Å range:
|
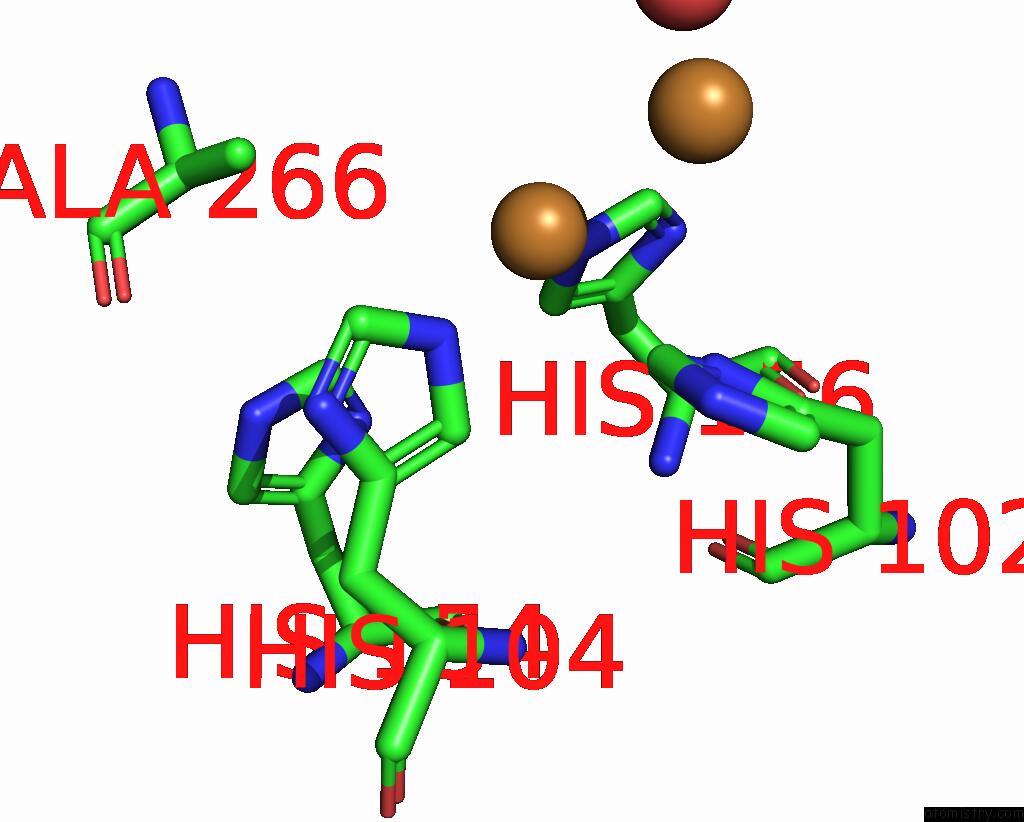
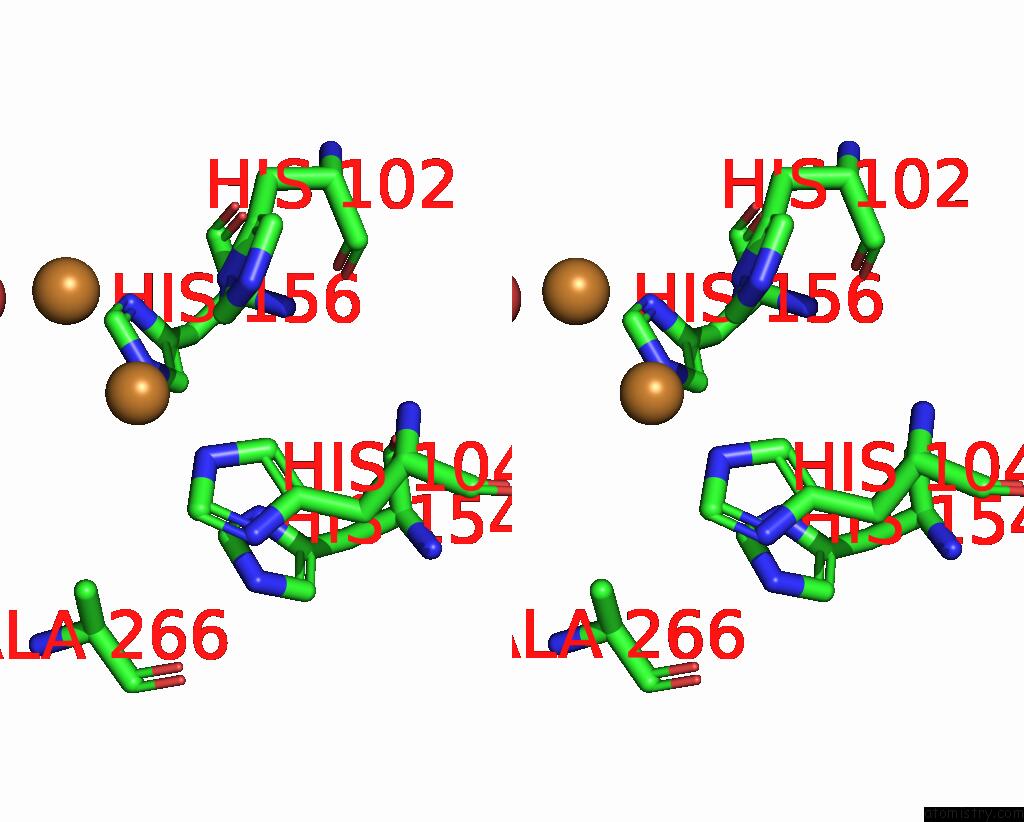
Copper binding site 4 out of 6 in 7bdn
Go back to
Copper binding site 4 out
of 6 in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form within 5.0Å range:
|
Copper binding site 5 out of 6 in 7bdn
Go back to
Copper binding site 5 out
of 6 in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form

Mono view
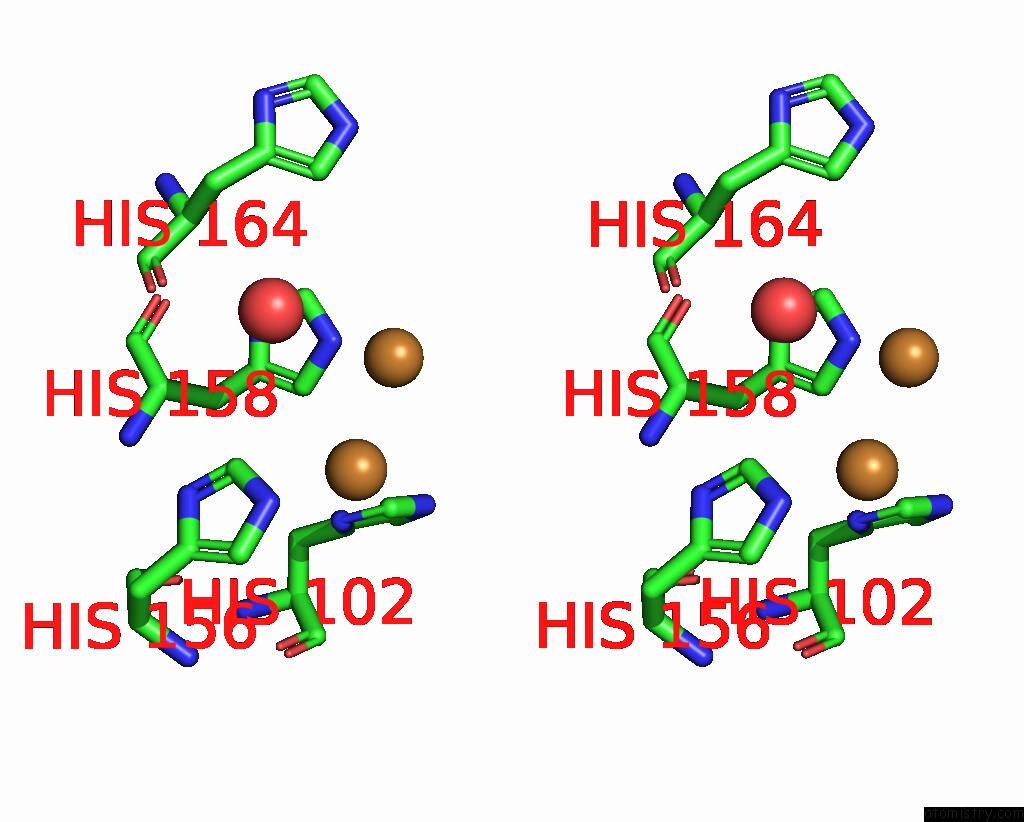
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form within 5.0Å range:
|
Copper binding site 6 out of 6 in 7bdn
Go back to
Copper binding site 6 out
of 6 in the Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form
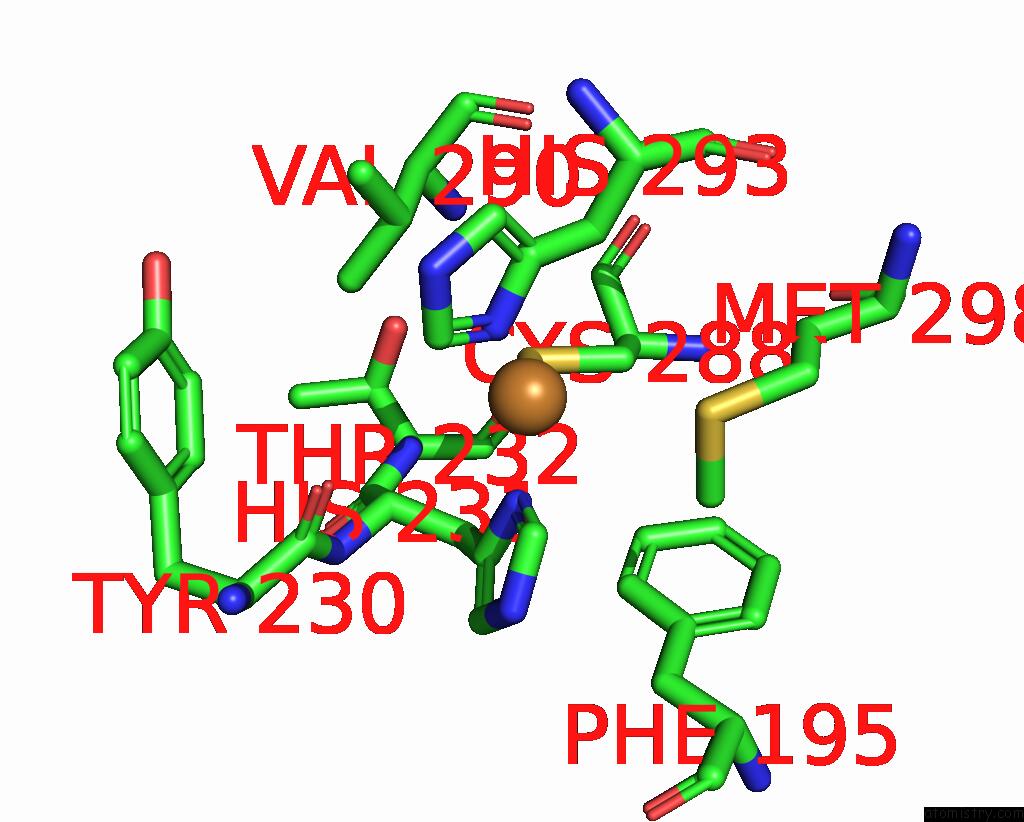
Mono view
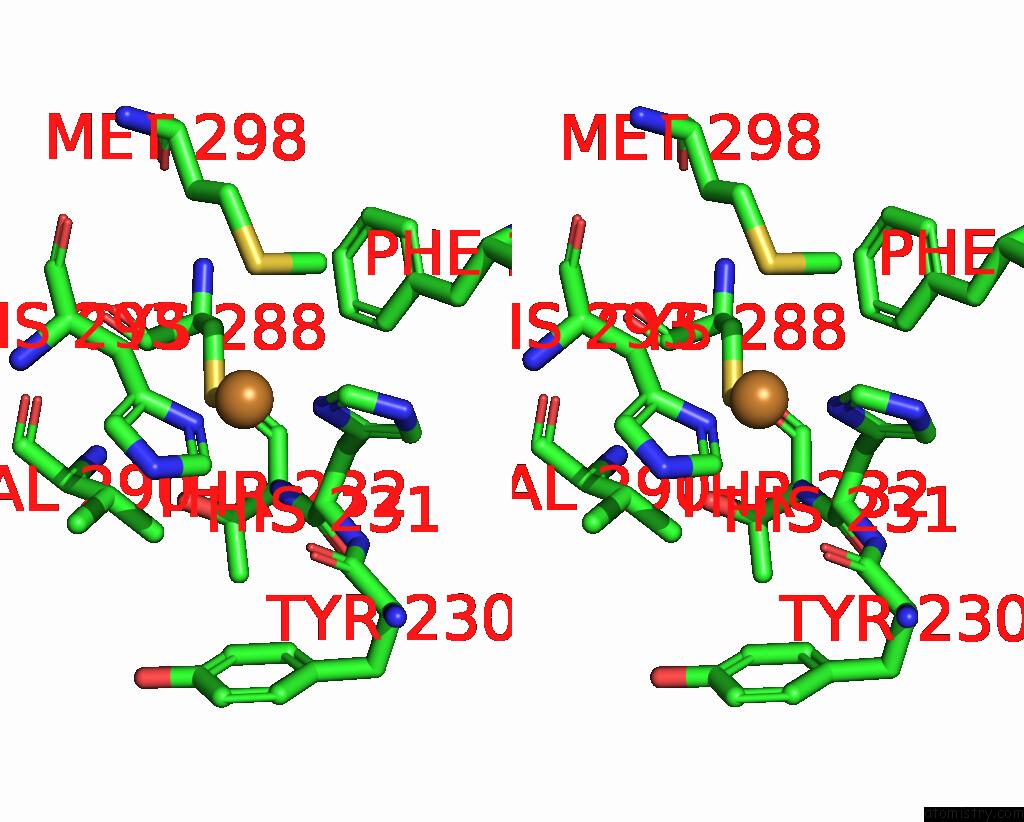
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Structure of the Streptomyces Coelicolor Small Laccase - Cubic Crystal Form within 5.0Å range:
|
Reference:
K.Zovo,
H.Pupart,
A.Van Wieren,
R.E.Gillilan,
Q.Huang,
S.Majumdar,
T.Lukk.
Substitution of the Methionine Axial Ligand of the T1 Copper For the Fungal-Like Phenylalanine Ligand (M298F) Causes Local Structural Perturbations That Lead to Thermal Instability and Reduced Catalytic Efficiency of the Small Laccase From Streptomyces Coelicolor A3(2). Acs Omega V. 7 6184 2022.
ISSN: ESSN 2470-1343
PubMed: 35224382
DOI: 10.1021/ACSOMEGA.1C06668
Page generated: Wed Jul 31 08:22:01 2024
ISSN: ESSN 2470-1343
PubMed: 35224382
DOI: 10.1021/ACSOMEGA.1C06668
Last articles
Cl in 5AKFCl in 5AKP
Cl in 5AK6
Cl in 5AJQ
Cl in 5AHJ
Cl in 5AJK
Cl in 5AHY
Cl in 5AIB
Cl in 5AHW
Cl in 5AHI