Copper »
PDB 6ied-6l58 »
6l58 »
Copper in PDB 6l58: Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
Enzymatic activity of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
All present enzymatic activity of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II):
1.16.3.1;
1.16.3.1;
Protein crystallography data
The structure of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II), PDB code: 6l58
was solved by
Q.Q.Jiang,
X.R.Su,
T.H.Ming,
H.S.Huan,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.40 / 3.90 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 217.288, 217.288, 134.083, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.7 / 26.4 |
Copper Binding Sites:
The binding sites of Copper atom in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
(pdb code 6l58). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 8 binding sites of Copper where determined in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II), PDB code: 6l58:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Copper where determined in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II), PDB code: 6l58:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Copper binding site 1 out of 8 in 6l58
Go back to
Copper binding site 1 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
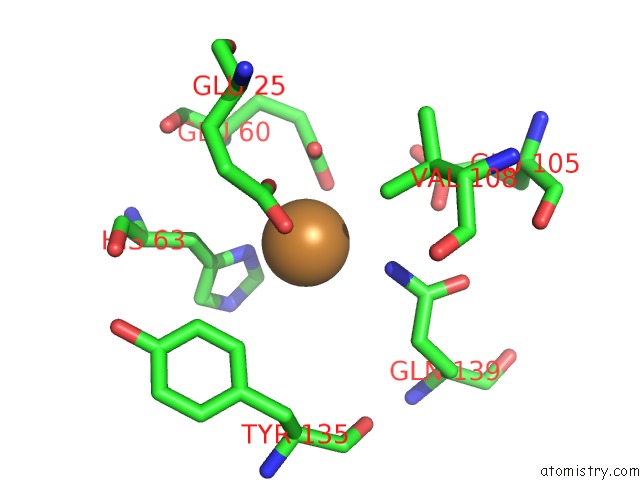
Mono view
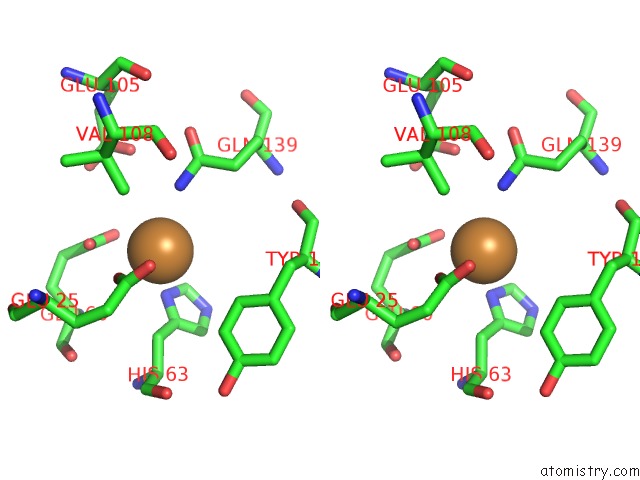
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
Copper binding site 2 out of 8 in 6l58
Go back to
Copper binding site 2 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
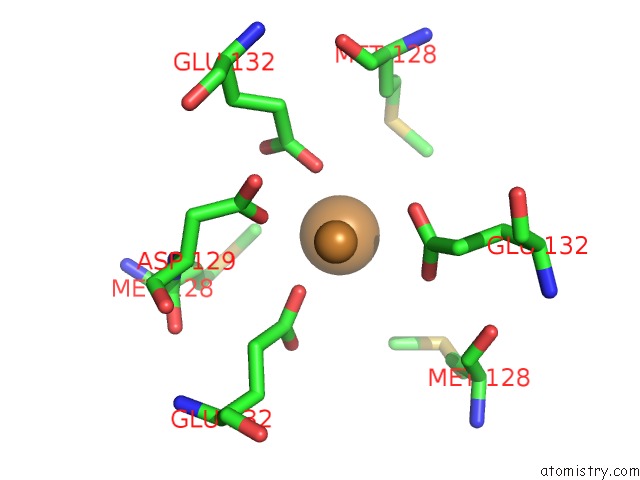
Mono view
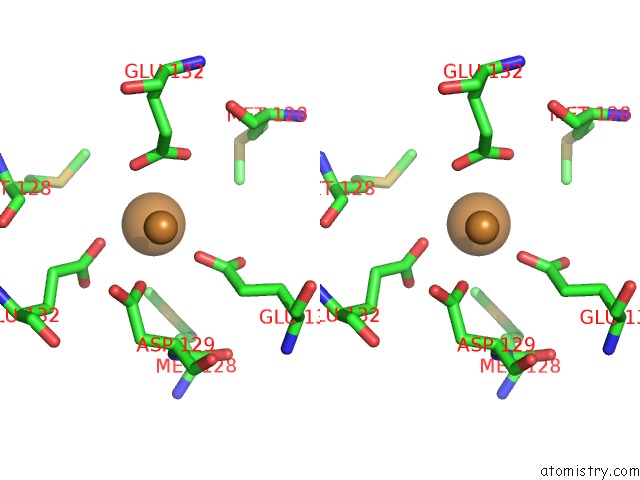
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
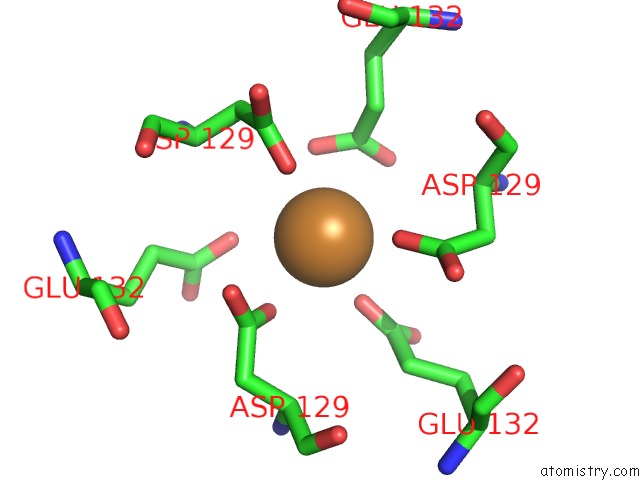
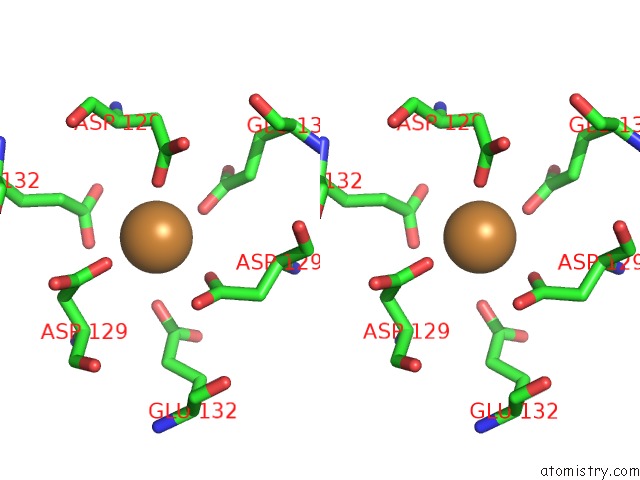
Copper binding site 3 out of 8 in 6l58
Go back to
Copper binding site 3 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
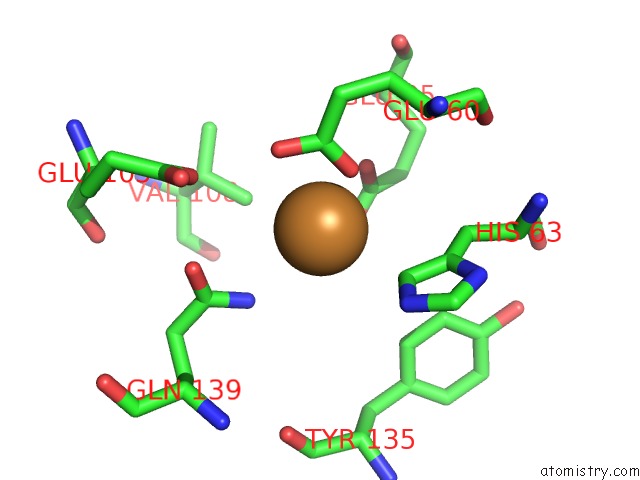
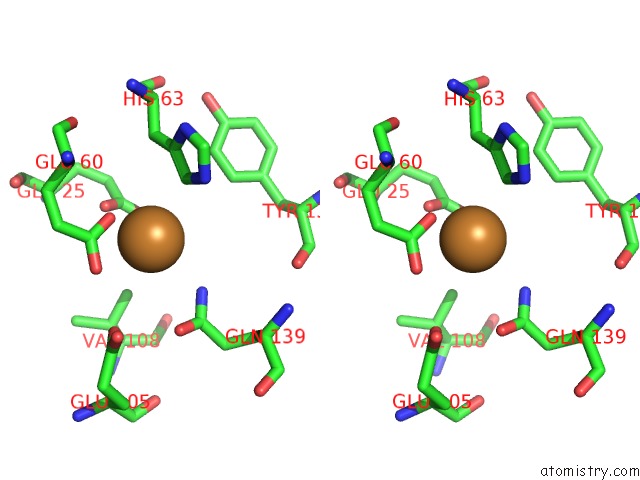
Copper binding site 4 out of 8 in 6l58
Go back to
Copper binding site 4 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
Copper binding site 5 out of 8 in 6l58
Go back to
Copper binding site 5 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
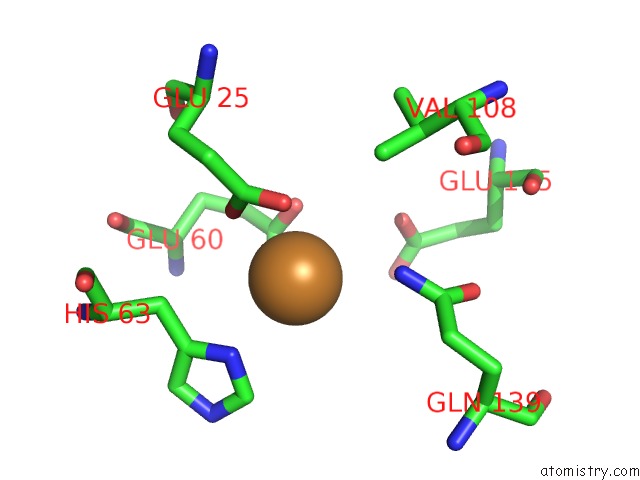
Mono view
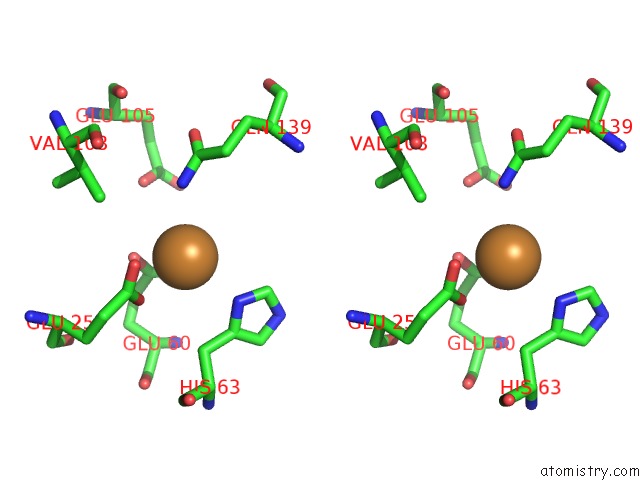
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
Copper binding site 6 out of 8 in 6l58
Go back to
Copper binding site 6 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
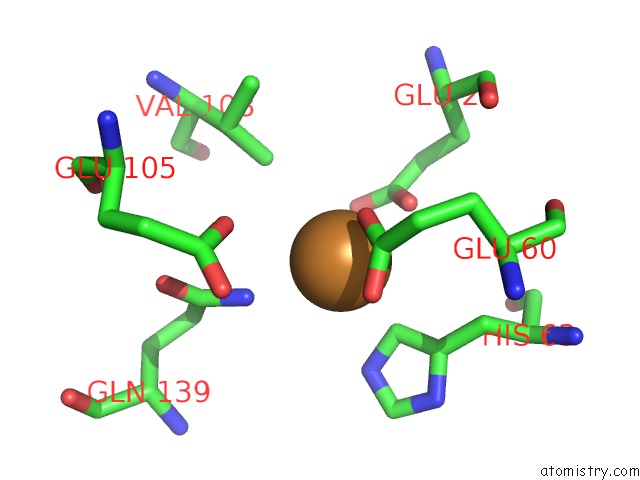
Mono view
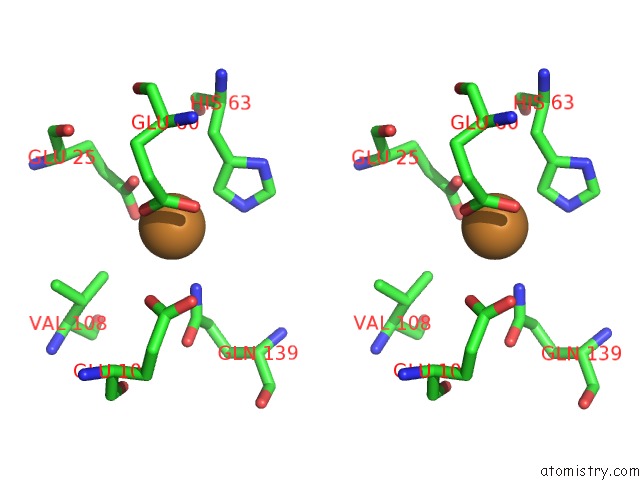
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
Copper binding site 7 out of 8 in 6l58
Go back to
Copper binding site 7 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
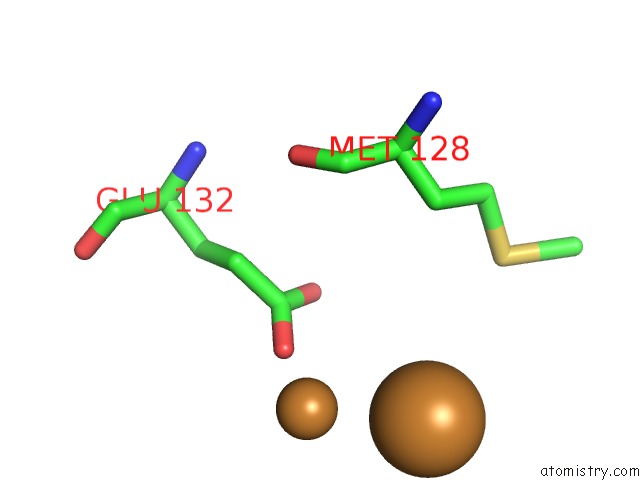
Mono view
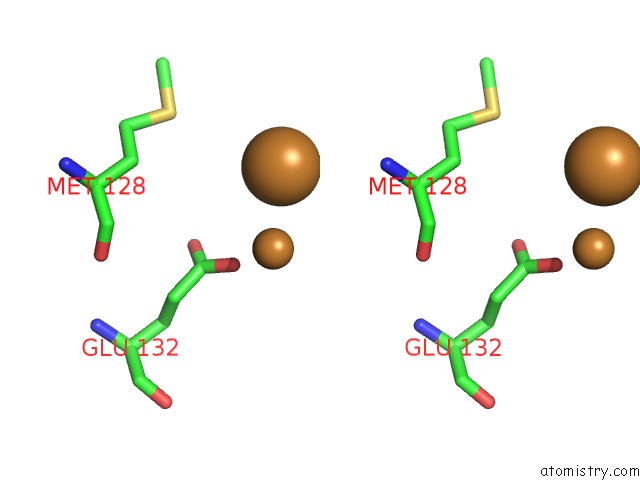
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 7 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
Copper binding site 8 out of 8 in 6l58
Go back to
Copper binding site 8 out
of 8 in the Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II)
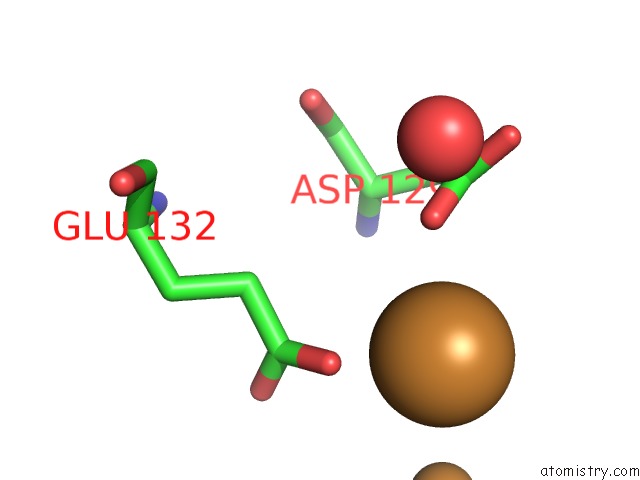
Mono view
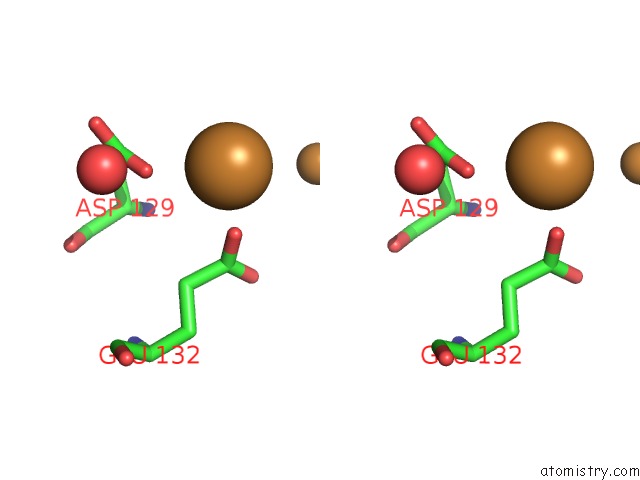
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 8 of Cu(II) Loaded Tegillarca Granosa M-Ferritin Soaked with Fe(II) within 5.0Å range:
|
Reference:
Q.Q.Jiang,
X.R.Su,
T.H.Ming,
H.S.Huan.
Crystal Structure Analysis Unravels How CU2+ in CU2+-Ferritin Nanocage Perturb the Functions of Tegillarca Granosa Ferritin To Be Published.
Page generated: Mon Jul 14 06:29:48 2025
Last articles
F in 4IBIF in 4IAH
F in 4IAE
F in 4I9H
F in 4I9N
F in 4I9O
F in 4IA9
F in 4I89
F in 4I7S
F in 4I87