Copper »
PDB 5zpp-6fja »
6cxh »
Copper in PDB 6cxh: Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z
Enzymatic activity of Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z
All present enzymatic activity of Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z:
1.14.13.25;
1.14.13.25;
Protein crystallography data
The structure of Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z, PDB code: 6cxh
was solved by
S.Y.Ro,
A.C.Rosenzweig,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.58 / 2.70 |
| Space group | P 63 |
| Cell size a, b, c (Å), α, β, γ (°) | 143.840, 143.840, 146.152, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 21.3 / 26.8 |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z
(pdb code 6cxh). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total only one binding site of Copper was determined in the Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z, PDB code: 6cxh:
In total only one binding site of Copper was determined in the Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z, PDB code: 6cxh:
Copper binding site 1 out of 1 in 6cxh
Go back to
Copper binding site 1 out
of 1 in the Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z
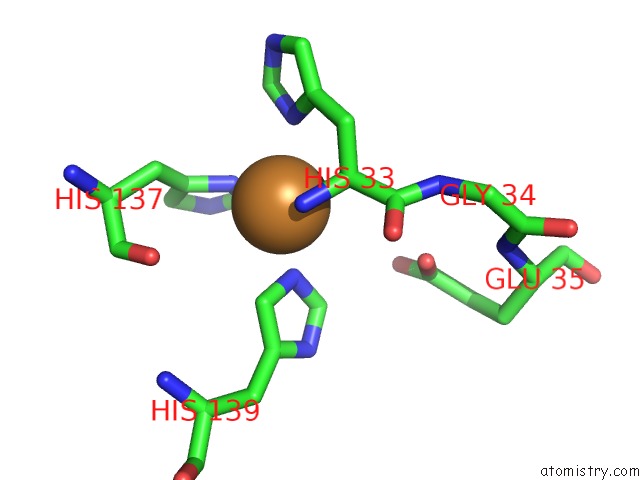
Mono view
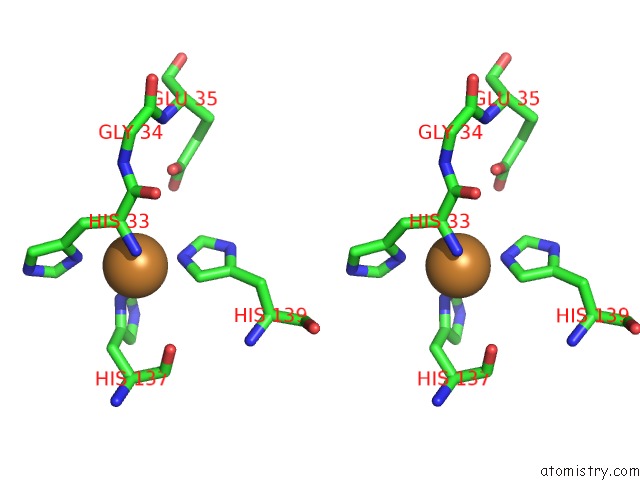
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of Particulate Methane Monooxygenase From Methylomicrobium Alcaliphilum 20Z within 5.0Å range:
|
Reference:
S.Y.Ro,
M.O.Ross,
Y.W.Deng,
S.Batelu,
T.J.Lawton,
J.D.Hurley,
T.L.Stemmler,
B.M.Hoffman,
A.C.Rosenzweig.
From Micelles to Bicelles: Effect of the Membrane on Particulate Methane Monooxygenase Activity. J. Biol. Chem. V. 293 10457 2018.
ISSN: ESSN 1083-351X
PubMed: 29739854
DOI: 10.1074/JBC.RA118.003348
Page generated: Mon Jul 14 05:55:06 2025
ISSN: ESSN 1083-351X
PubMed: 29739854
DOI: 10.1074/JBC.RA118.003348
Last articles
Fe in 1XLOFe in 1XLN
Fe in 1XK3
Fe in 1XK2
Fe in 1XK1
Fe in 1XKW
Fe in 1XK0
Fe in 1XJZ
Fe in 1XJ6
Fe in 1XJ4