Copper »
PDB 5z86-5zpo »
5zp0 »
Copper in PDB 5zp0: Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2)
Enzymatic activity of Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2)
All present enzymatic activity of Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2):
1.4.3.21;
1.4.3.21;
Protein crystallography data
The structure of Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2), PDB code: 5zp0
was solved by
T.Murakawa,
S.Baba,
Y.Kawano,
H.Hayashi,
T.Yano,
K.Tanizawa,
T.Kumasaka,
M.Yamamoto,
T.Okajima,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.68 / 1.74 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 193.986, 65.077, 158.957, 90.00, 117.18, 90.00 |
| R / Rfree (%) | 15 / 17.7 |
Other elements in 5zp0:
The structure of Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2) also contains other interesting chemical elements:
| Sodium | (Na) | 3 atoms |
Copper Binding Sites:
The binding sites of Copper atom in the Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2)
(pdb code 5zp0). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2), PDB code: 5zp0:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2), PDB code: 5zp0:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 5zp0
Go back to
Copper binding site 1 out
of 2 in the Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2)
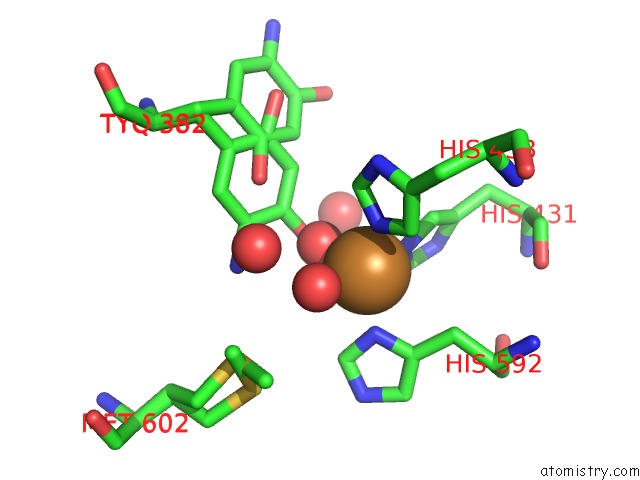
Mono view
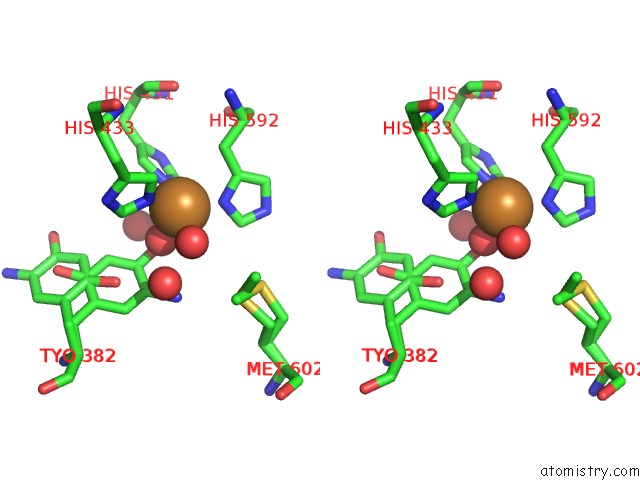
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2) within 5.0Å range:
|
Copper binding site 2 out of 2 in 5zp0
Go back to
Copper binding site 2 out
of 2 in the Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2)
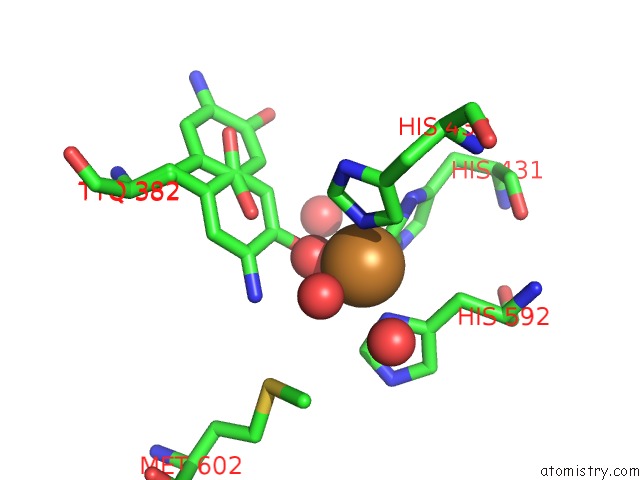
Mono view
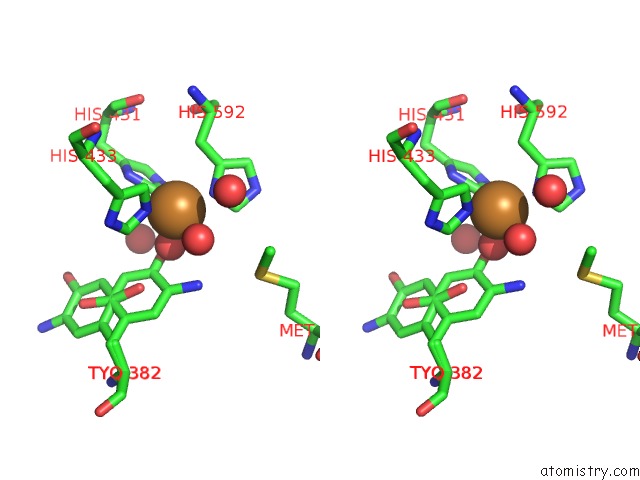
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Copper Amine Oxidase From Arthrobacter Globiformis Anaerobically Reduced By Ethylamine at pH 8 at 288 K (2) within 5.0Å range:
|
Reference:
T.Murakawa,
S.Baba,
Y.Kawano,
H.Hayashi,
T.Yano,
T.Kumasaka,
M.Yamamoto,
K.Tanizawa,
T.Okajima.
In Crystallothermodynamic Analysis of Conformational Change of the Topaquinone Cofactor in Bacterial Copper Amine Oxidase. Proc. Natl. Acad. Sci. V. 116 135 2019U.S.A..
ISSN: ESSN 1091-6490
PubMed: 30563857
DOI: 10.1073/PNAS.1811837116
Page generated: Mon Jul 14 05:48:27 2025
ISSN: ESSN 1091-6490
PubMed: 30563857
DOI: 10.1073/PNAS.1811837116
Last articles
F in 7N77F in 7N7W
F in 7N73
F in 7N7L
F in 7N72
F in 7N70
F in 7N3J
F in 7N6F
F in 7N66
F in 7N33