Copper »
PDB 5onx-5wbc »
5tki »
Copper in PDB 5tki: Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement
Protein crystallography data
The structure of Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement, PDB code: 5tki
was solved by
W.B.O'dell,
F.Meilleur,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | N/A / 1.50 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 68.120, 42.230, 70.290, 90.00, 98.33, 90.00 |
| R / Rfree (%) | 21.6 / 25.3 |
Copper Binding Sites:
The binding sites of Copper atom in the Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement
(pdb code 5tki). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement, PDB code: 5tki:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement, PDB code: 5tki:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 5tki
Go back to
Copper binding site 1 out
of 2 in the Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement
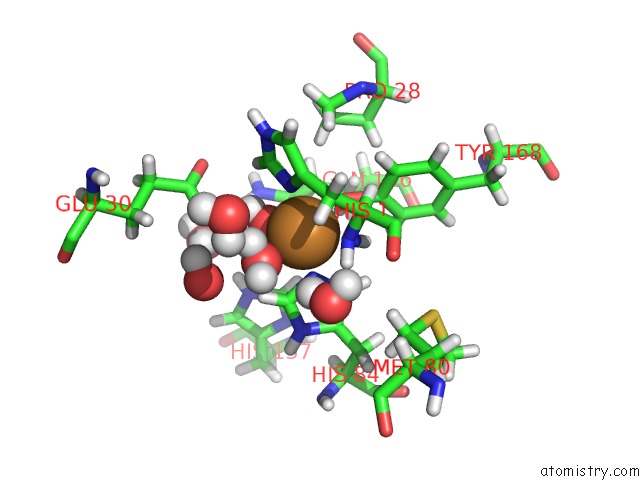
Mono view
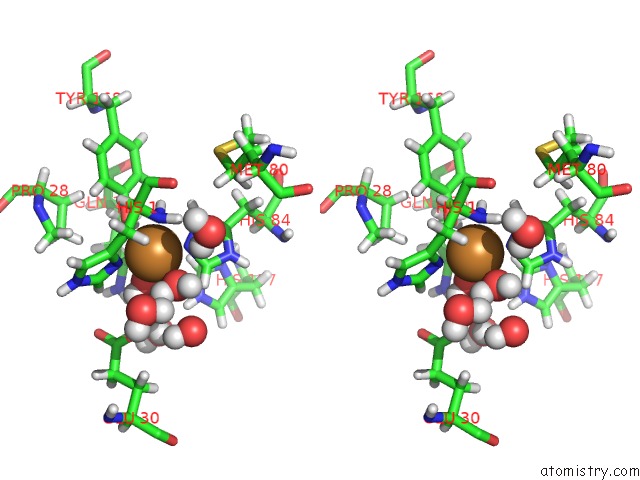
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement within 5.0Å range:
|
Copper binding site 2 out of 2 in 5tki
Go back to
Copper binding site 2 out
of 2 in the Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement
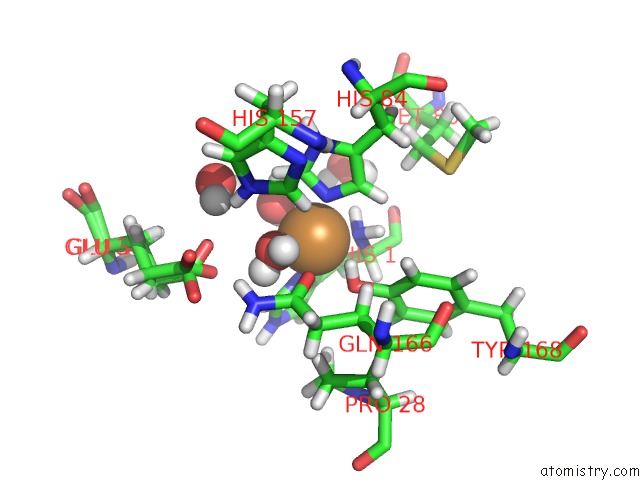
Mono view
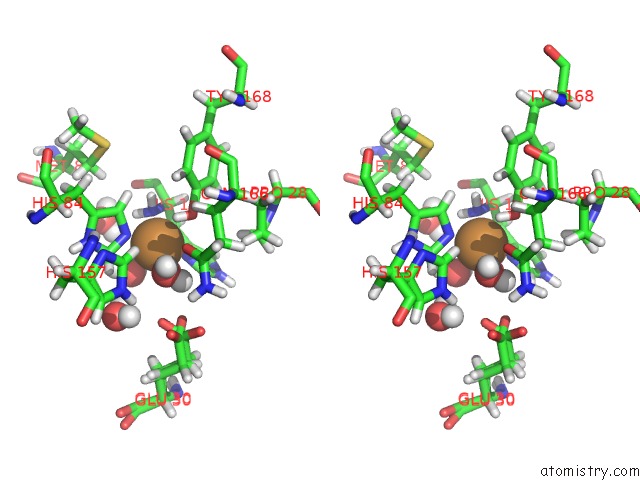
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Neurospora Crassa Polysaccharide Monooxygenase 2 Resting State Joint X-Ray/Neutron Refinement within 5.0Å range:
|
Reference:
W.B.O'dell,
P.K.Agarwal,
F.Meilleur.
Oxygen Activation at the Active Site of A Fungal Lytic Polysaccharide Monooxygenase. Angew. Chem. Int. Ed. Engl. V. 56 767 2017.
ISSN: ESSN 1521-3773
PubMed: 28004877
DOI: 10.1002/ANIE.201610502
Page generated: Wed Jul 31 05:10:37 2024
ISSN: ESSN 1521-3773
PubMed: 28004877
DOI: 10.1002/ANIE.201610502
Last articles
Cl in 7WAOCl in 7WBF
Cl in 7WBE
Cl in 7WBD
Cl in 7WB9
Cl in 7WAL
Cl in 7WAK
Cl in 7WAM
Cl in 7WAN
Cl in 7W9R