Copper »
PDB 5ce9-5i0y »
5ftz »
Copper in PDB 5ftz: AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans
Protein crystallography data
The structure of AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans, PDB code: 5ftz
was solved by
A.K.C.Chaplin,
M.T.Wilson,
M.A.Hough,
D.A.Svistunenko,
G.R.Hemsworth,
P.H.Walton,
E.Vijgenboom,
J.A.R.Worrall,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 60.76 / 1.38 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 69.620, 32.430, 61.320, 90.00, 97.77, 90.00 |
| R / Rfree (%) | 13.504 / 15.999 |
Copper Binding Sites:
The binding sites of Copper atom in the AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans
(pdb code 5ftz). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans, PDB code: 5ftz:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans, PDB code: 5ftz:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 5ftz
Go back to
Copper binding site 1 out
of 2 in the AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans
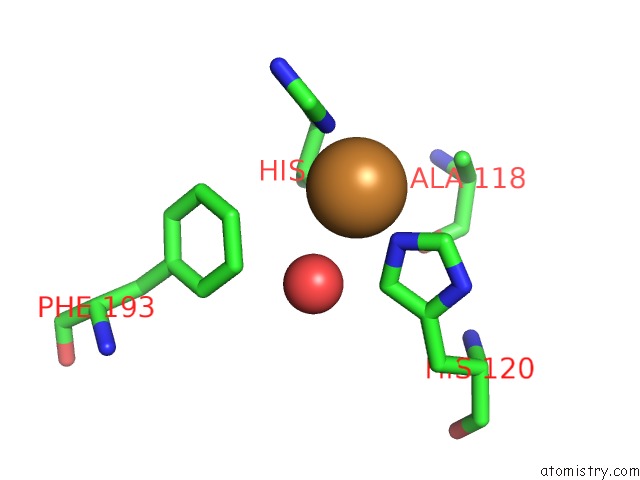
Mono view
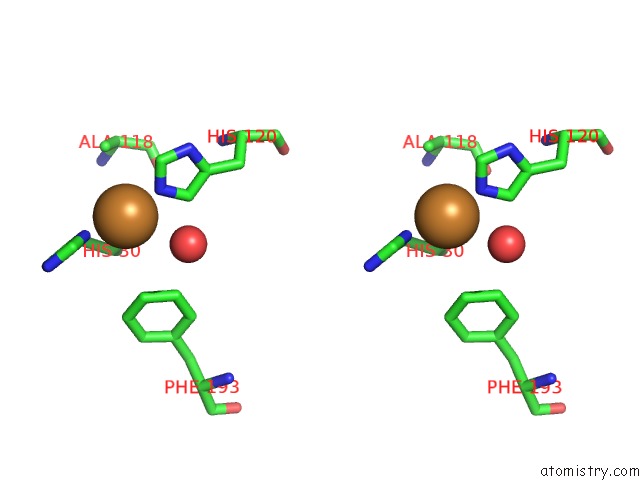
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans within 5.0Å range:
|
Copper binding site 2 out of 2 in 5ftz
Go back to
Copper binding site 2 out
of 2 in the AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans
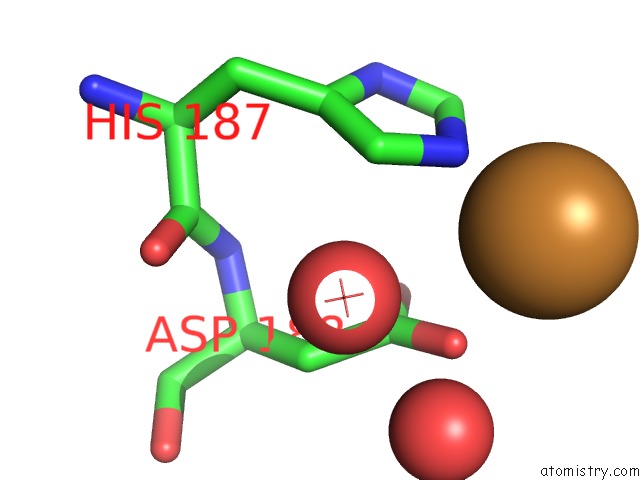
Mono view
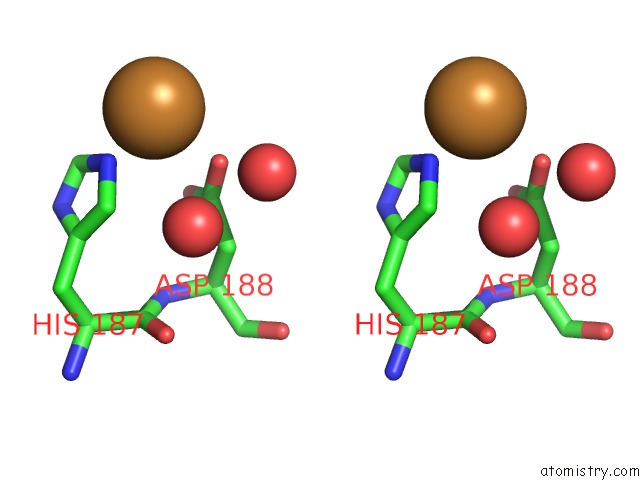
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of AA10 Lytic Polysaccharide Monooxygenase (Lpmo) From Streptomyces Lividans within 5.0Å range:
|
Reference:
A.K.Chaplin,
M.T.Wilson,
M.A.Hough,
D.A.Svistunenko,
G.R.Hemsworth,
P.H.Walton,
E.Vijgenboom,
J.A.R.Worrall.
Heterogeneity in the Histidine-Brace Copper Coordination Sphere in AA10 Lytic Polysaccharide Monooxygenases. J.Biol.Chem. V. 291 12838 2016.
ISSN: ISSN 0021-9258
PubMed: 27129229
DOI: 10.1074/JBC.M116.722447
Page generated: Mon Jul 14 04:35:10 2025
ISSN: ISSN 0021-9258
PubMed: 27129229
DOI: 10.1074/JBC.M116.722447
Last articles
F in 4DHPF in 4DBU
F in 4DHM
F in 4DEB
F in 4DC3
F in 4D8C
F in 4D83
F in 4DBQ
F in 4DBN
F in 4DAN