Copper »
PDB 4tm7-4yst »
4w7e »
Copper in PDB 4w7e: Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group
Protein crystallography data
The structure of Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group, PDB code: 4w7e
was solved by
D.J.Leibly,
G.S.Waldo,
T.O.Yeates,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 67.92 / 2.59 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 96.050, 96.050, 69.960, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.7 / 26.2 |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group
(pdb code 4w7e). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total only one binding site of Copper was determined in the Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group, PDB code: 4w7e:
In total only one binding site of Copper was determined in the Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group, PDB code: 4w7e:
Copper binding site 1 out of 1 in 4w7e
Go back to
Copper binding site 1 out
of 1 in the Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group

Mono view
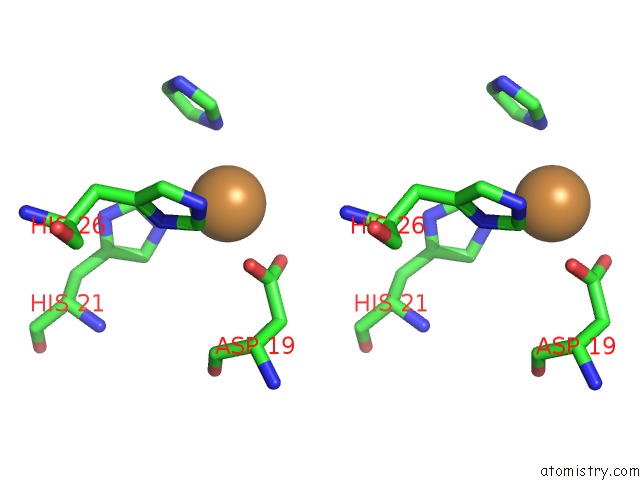
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of Full-Length Split Gfp Mutant E124H/K126H with Copper Mediated Crystal Contacts, P 41 21 2 Space Group within 5.0Å range:
|
Reference:
D.J.Leibly,
M.A.Arbing,
I.Pashkov,
N.Devore,
G.S.Waldo,
T.C.Terwilliger,
T.O.Yeates.
Engineering Novel Oligomeric Gfp Molecules For Synthetic Symmetrization Applications To Be Published.
Page generated: Mon Jul 14 04:08:15 2025
Last articles
Fe in 1O94Fe in 1O9R
Fe in 1O9X
Fe in 1O7P
Fe in 1O7W
Fe in 1O7N
Fe in 1O7H
Fe in 1O7G
Fe in 1O7M
Fe in 1O1P