Copper »
PDB 4mfh-4rkn »
4pi0 »
Copper in PDB 4pi0: Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
Enzymatic activity of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
All present enzymatic activity of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper:
1.14.18.3;
1.14.18.3;
Protein crystallography data
The structure of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper, PDB code: 4pi0
was solved by
S.Sirajuddin,
A.C.Rosenzweig,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 3.15 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 116.361, 184.739, 188.637, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 22.1 / 28 |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
(pdb code 4pi0). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 6 binding sites of Copper where determined in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper, PDB code: 4pi0:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Copper where determined in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper, PDB code: 4pi0:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
Copper binding site 1 out of 6 in 4pi0
Go back to
Copper binding site 1 out
of 6 in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper within 5.0Å range:
|
Copper binding site 2 out of 6 in 4pi0
Go back to
Copper binding site 2 out
of 6 in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper within 5.0Å range:
|
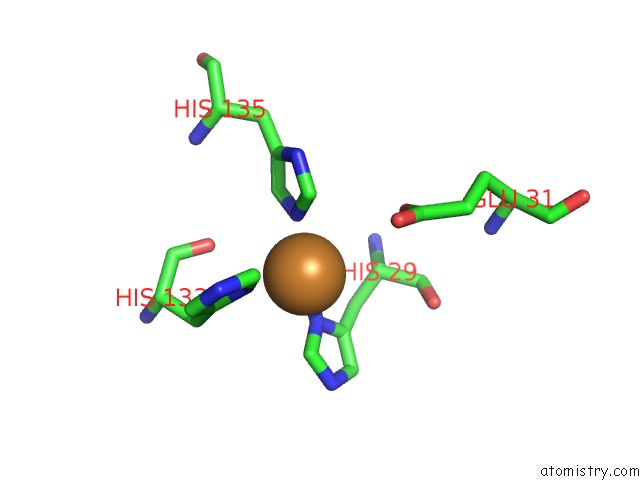
Copper binding site 3 out of 6 in 4pi0
Go back to
Copper binding site 3 out
of 6 in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper within 5.0Å range:
|
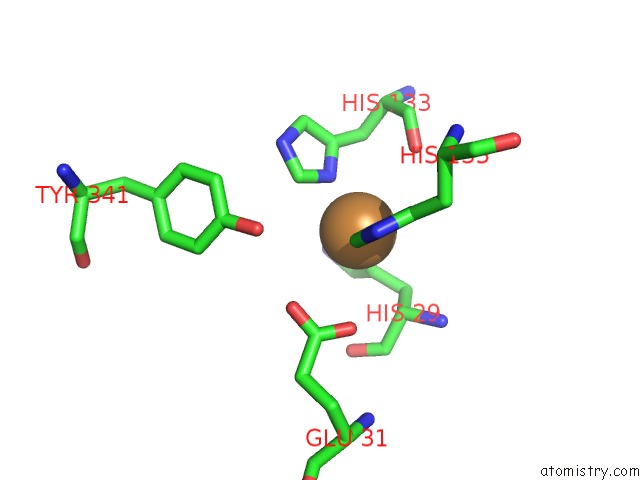
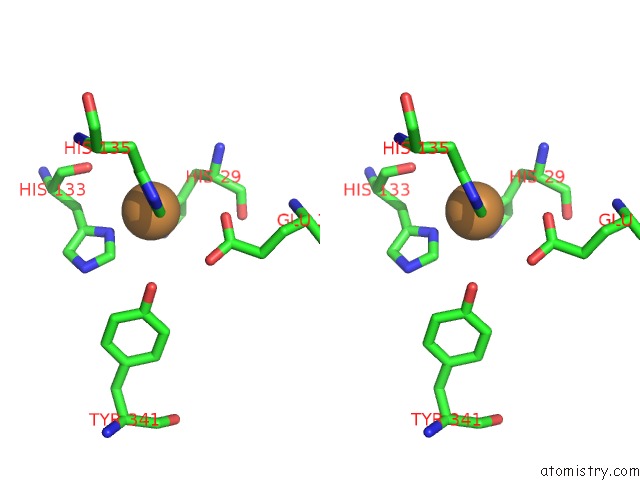
Copper binding site 4 out of 6 in 4pi0
Go back to
Copper binding site 4 out
of 6 in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper within 5.0Å range:
|
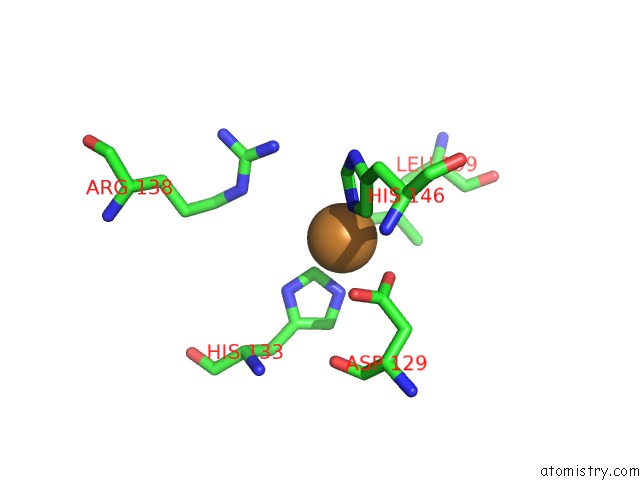
Copper binding site 5 out of 6 in 4pi0
Go back to
Copper binding site 5 out
of 6 in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
Mono view
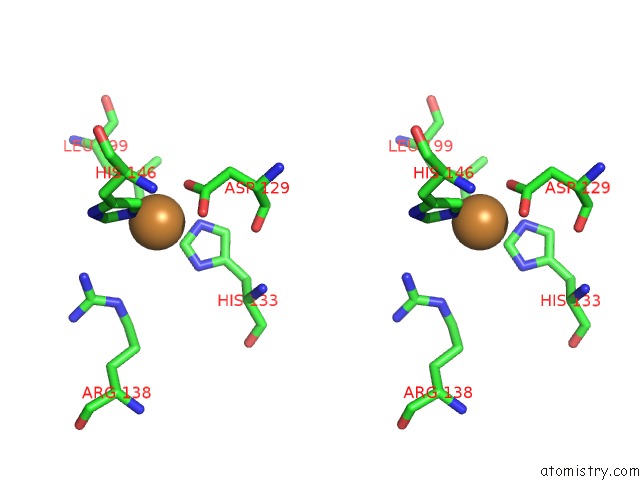
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper within 5.0Å range:
|
Copper binding site 6 out of 6 in 4pi0
Go back to
Copper binding site 6 out
of 6 in the Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper
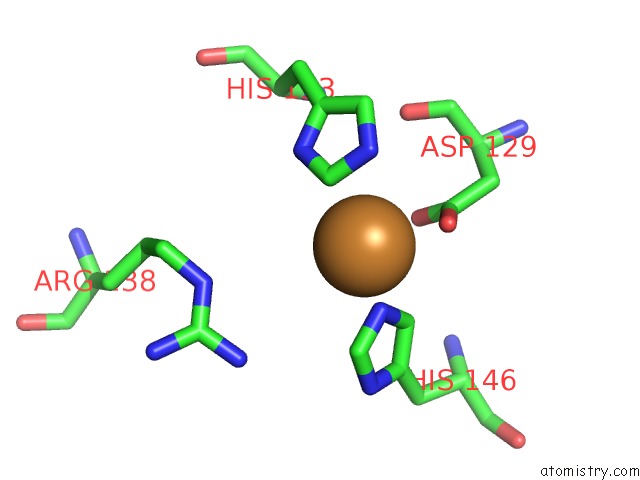
Mono view
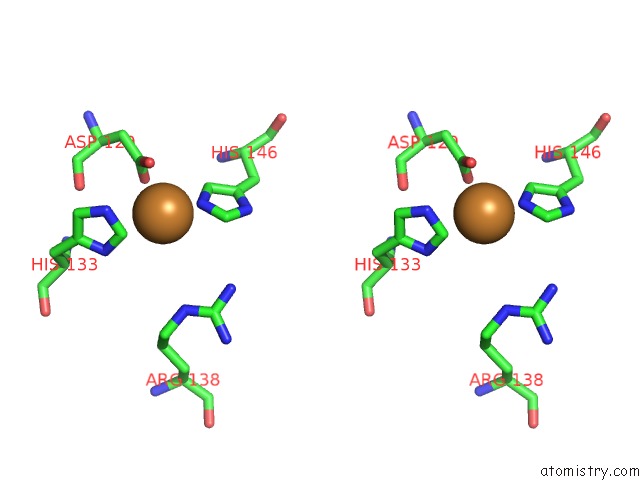
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Crystal Structure of Particulate Methane Monooxygenase From Methylocystis Sp. Atcc 49242 (Rockwell) Soaked in Copper within 5.0Å range:
|
Reference:
S.Sirajuddin,
D.Barupala,
S.Helling,
K.Marcus,
T.L.Stemmler,
A.C.Rosenzweig.
Effects of Zinc on Particulate Methane Monooxygenase Activity and Structure. J.Biol.Chem. V. 289 21782 2014.
ISSN: ESSN 1083-351X
PubMed: 24942740
DOI: 10.1074/JBC.M114.581363
Page generated: Wed Jul 31 03:23:01 2024
ISSN: ESSN 1083-351X
PubMed: 24942740
DOI: 10.1074/JBC.M114.581363
Last articles
Cl in 5QFACl in 5QEW
Cl in 5QEJ
Cl in 5QEV
Cl in 5QES
Cl in 5QEU
Cl in 5QDH
Cl in 5QEB
Cl in 5QE9
Cl in 5QCH