Copper »
PDB 4e9v-4hhg »
4eir »
Copper in PDB 4eir: Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2)
Protein crystallography data
The structure of Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2), PDB code: 4eir
was solved by
X.Li,
W.T.Beeson,
C.M.Phillips,
M.A.Marletta,
J.H.Cate,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 22.86 / 1.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 67.170, 41.990, 69.250, 90.00, 97.94, 90.00 |
| R / Rfree (%) | 13.2 / 14.9 |
Copper Binding Sites:
The binding sites of Copper atom in the Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2)
(pdb code 4eir). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2), PDB code: 4eir:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2), PDB code: 4eir:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 4eir
Go back to
Copper binding site 1 out
of 2 in the Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2)
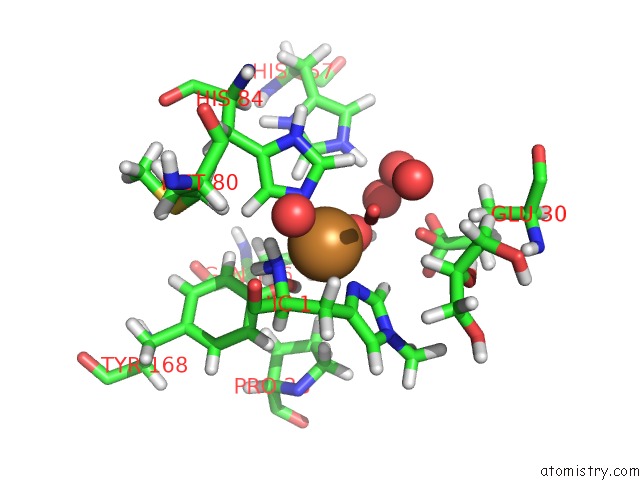
Mono view
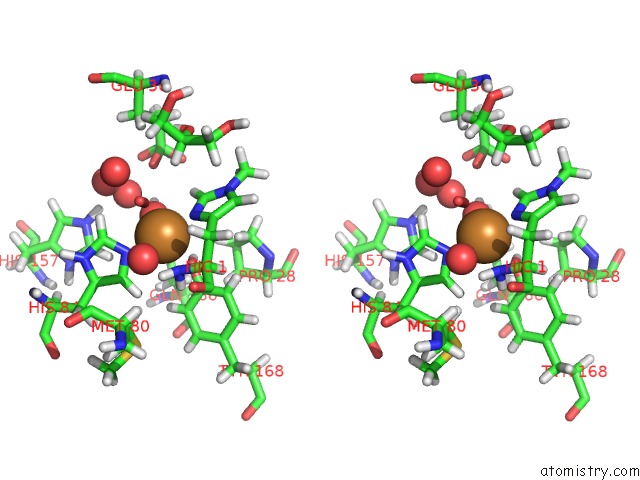
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2) within 5.0Å range:
|
Copper binding site 2 out of 2 in 4eir
Go back to
Copper binding site 2 out
of 2 in the Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2)
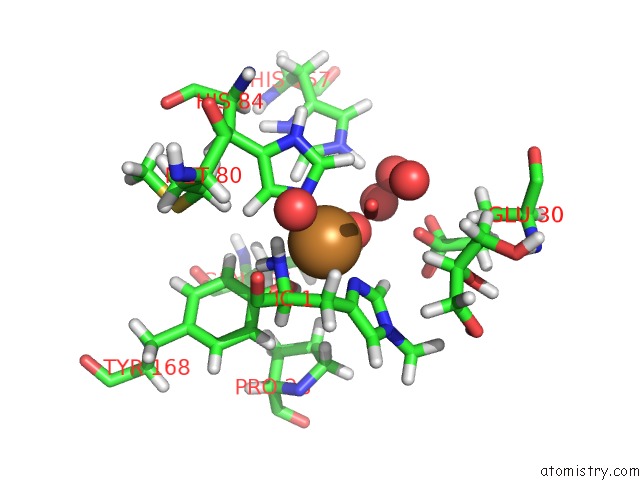
Mono view
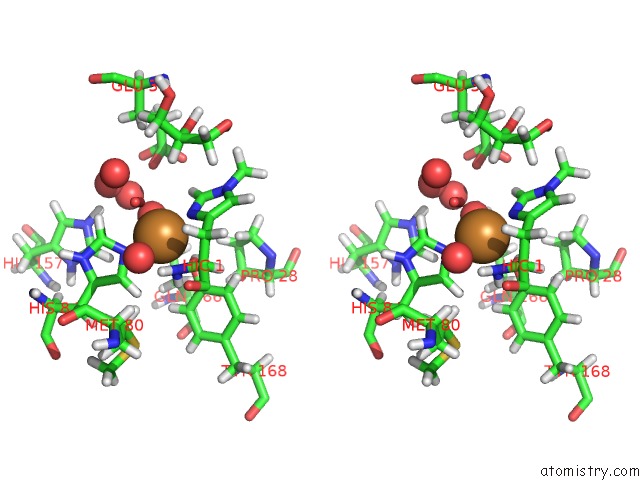
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases (Pmo-2) within 5.0Å range:
|
Reference:
X.Li,
W.T.Beeson,
C.M.Phillips,
M.A.Marletta,
J.H.Cate.
Structural Basis For Substrate Targeting and Catalysis By Fungal Polysaccharide Monooxygenases. Structure V. 20 1051 2012.
ISSN: ISSN 0969-2126
PubMed: 22578542
DOI: 10.1016/J.STR.2012.04.002
Page generated: Mon Jul 14 03:37:34 2025
ISSN: ISSN 0969-2126
PubMed: 22578542
DOI: 10.1016/J.STR.2012.04.002
Last articles
F in 4EWQF in 4EQU
F in 4EST
F in 4ENH
F in 4EPX
F in 4ENC
F in 4ENB
F in 4EMV
F in 4ENA
F in 4EN5