Copper »
PDB 4b61-4e9t »
4bed »
Copper in PDB 4bed: Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
Copper Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 30; Page 4, Binding sites: 31 - 32;Binding sites:
The binding sites of Copper atom in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units (pdb code 4bed). This binding sites where shown within 5.0 Angstroms radius around Copper atom.In total 32 binding sites of Copper where determined in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units, PDB code: 4bed:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Copper binding site 1 out of 32 in 4bed
Go back to
Copper binding site 1 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
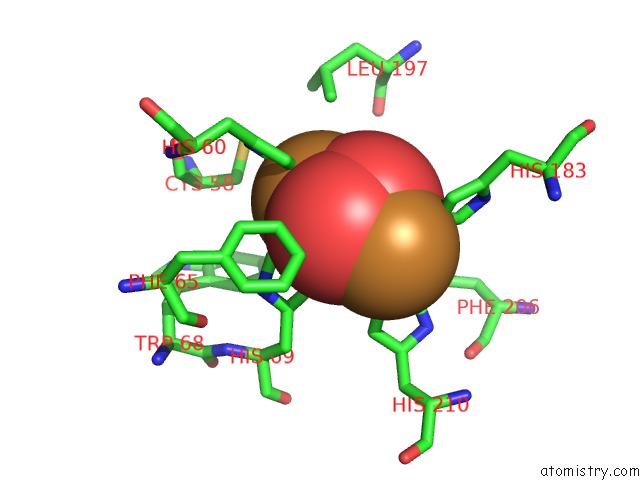
Mono view
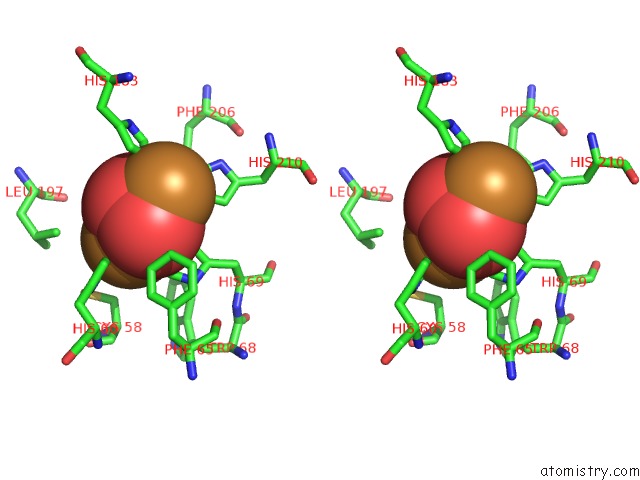
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 2 out of 32 in 4bed
Go back to
Copper binding site 2 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
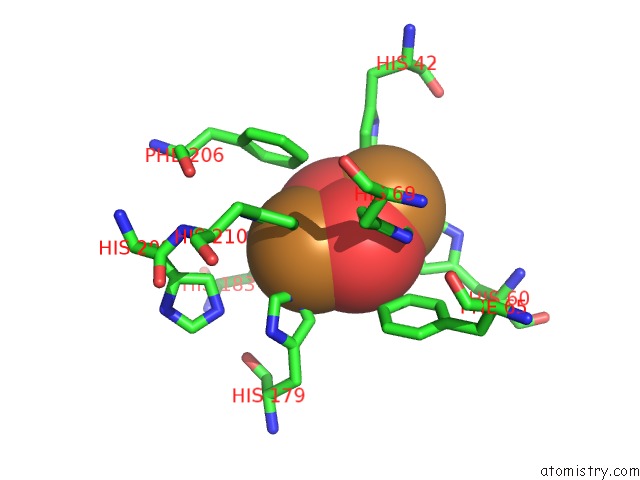
Mono view
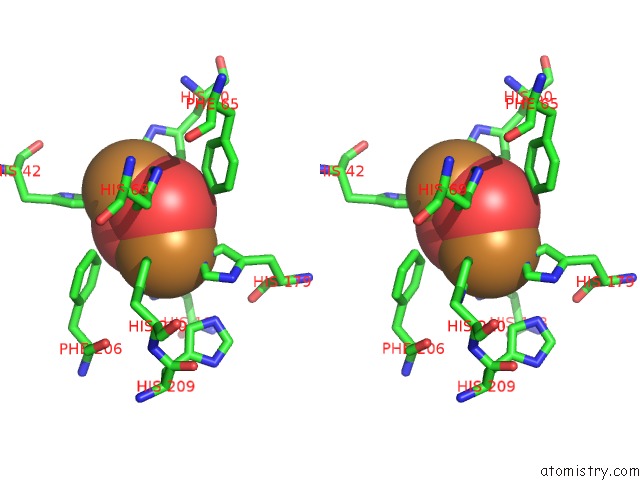
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 3 out of 32 in 4bed
Go back to
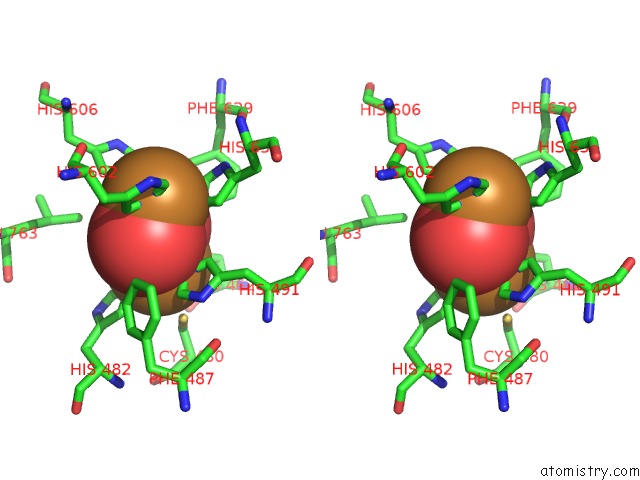
Copper binding site 3 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
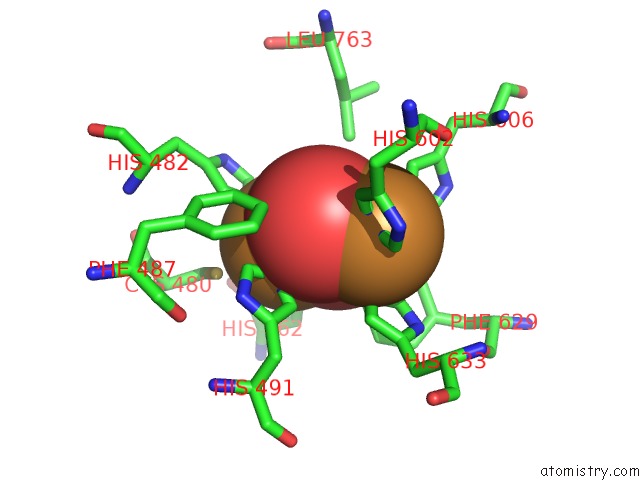
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 4 out of 32 in 4bed
Go back to
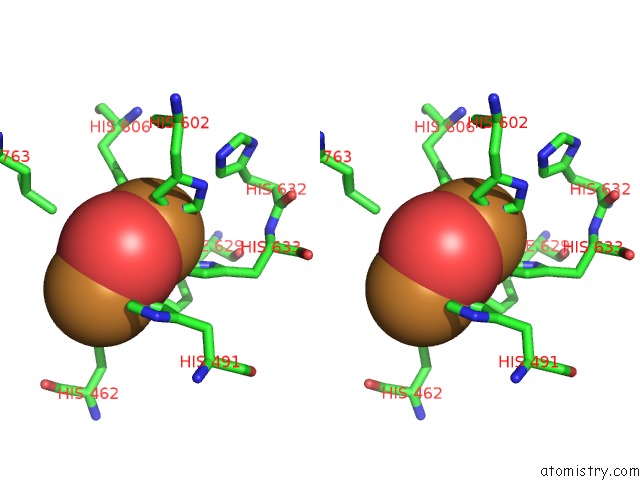
Copper binding site 4 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
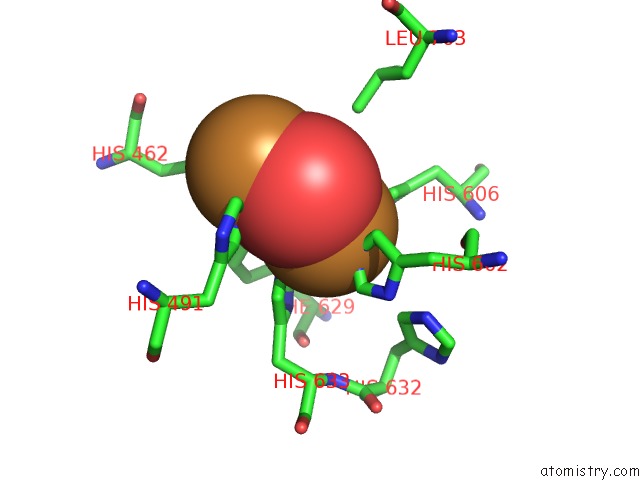
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 5 out of 32 in 4bed
Go back to
Copper binding site 5 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
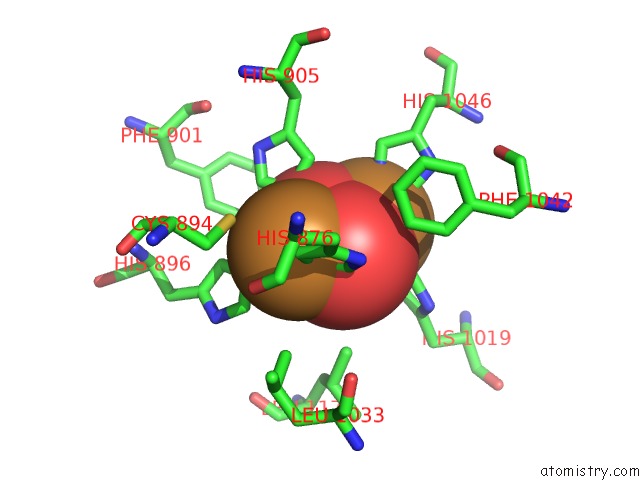
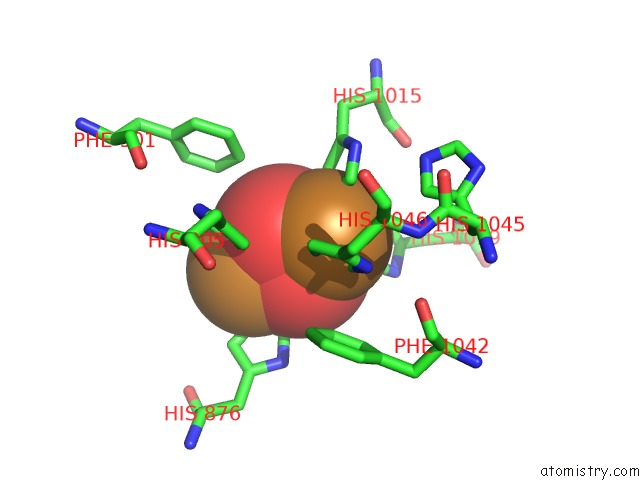
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 6 out of 32 in 4bed
Go back to
Copper binding site 6 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
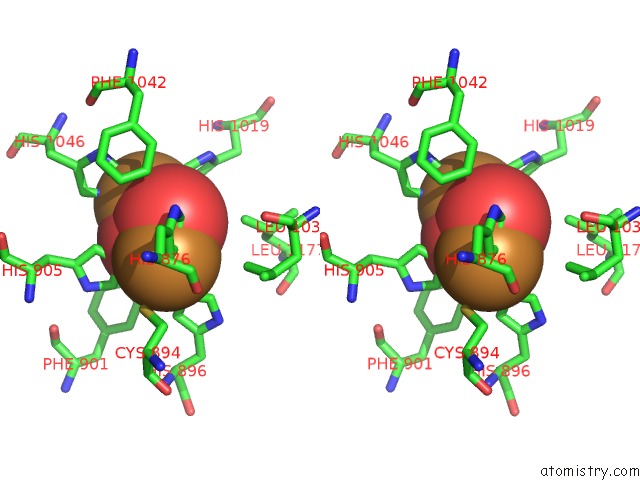
Mono view
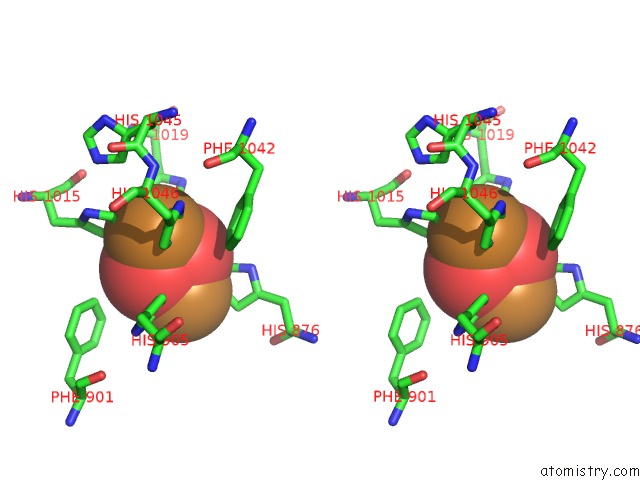
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 7 out of 32 in 4bed
Go back to
Copper binding site 7 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
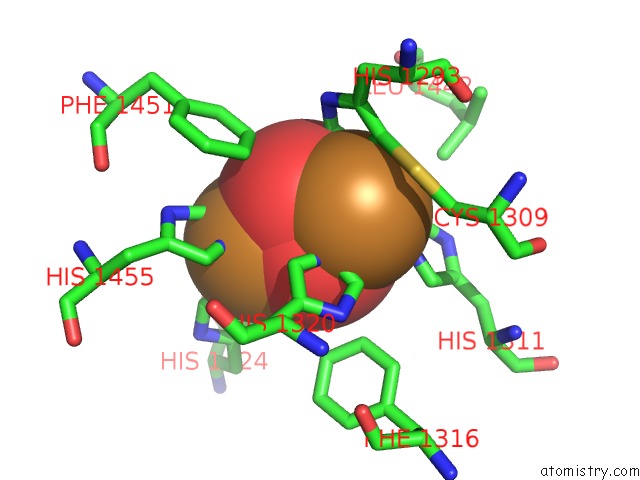
Mono view
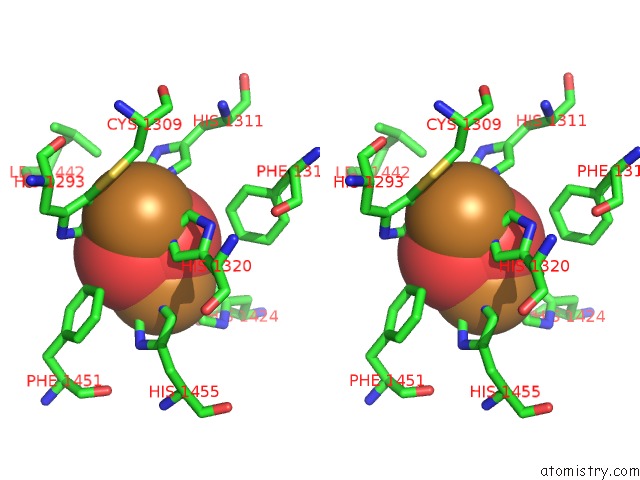
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 7 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 8 out of 32 in 4bed
Go back to
Copper binding site 8 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
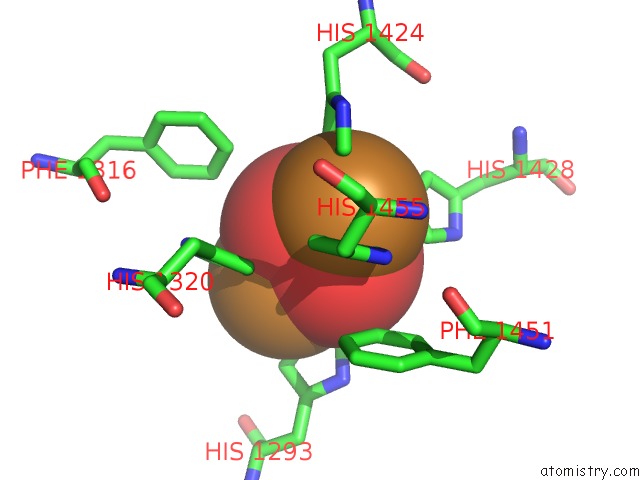
Mono view
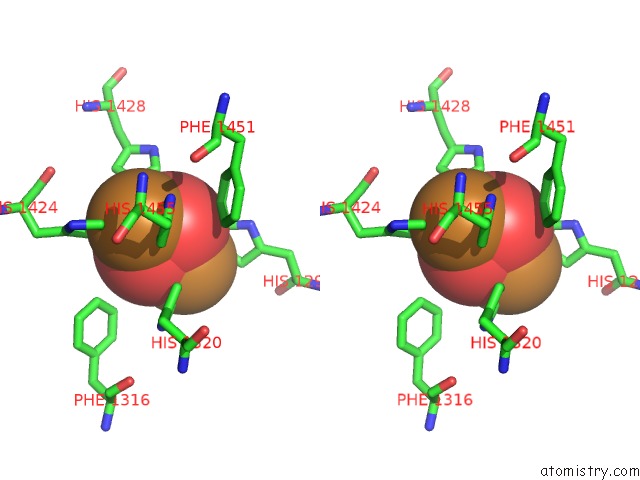
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 8 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 9 out of 32 in 4bed
Go back to
Copper binding site 9 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
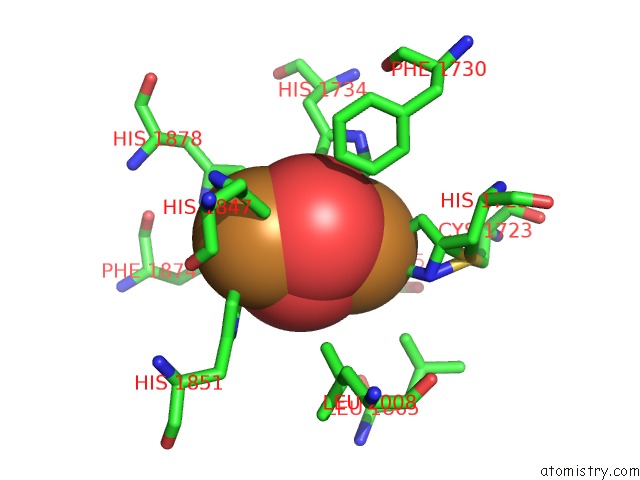
Mono view
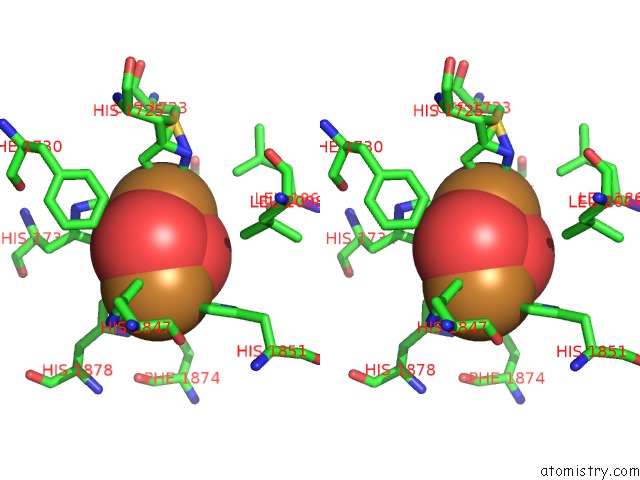
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 9 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Copper binding site 10 out of 32 in 4bed
Go back to
Copper binding site 10 out
of 32 in the Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units
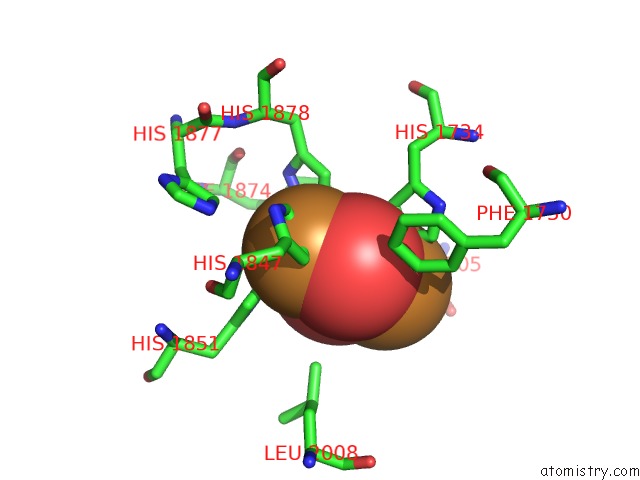
Mono view
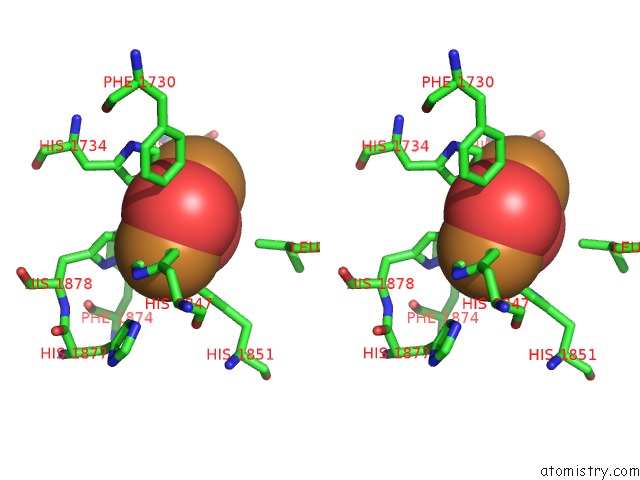
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 10 of Keyhole Limpet Hemocyanin (Klh): 9A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units within 5.0Å range:
|
Reference:
C.Gatsogiannis,
J.Markl.
Keyhole Limpet Hemocyanin: 9-A Cryoem Structure and Molecular Model of the KLH1 Didecamer Reveal the Interfaces and Intricate Topology of the 160 Functional Units. J.Mol.Biol. V. 385 963 2009.
ISSN: ISSN 0022-2836
PubMed: 19013468
DOI: 10.1016/J.JMB.2008.10.080
Page generated: Mon Jul 14 03:25:59 2025
ISSN: ISSN 0022-2836
PubMed: 19013468
DOI: 10.1016/J.JMB.2008.10.080
Last articles
Fe in 2B3YFe in 2B24
Fe in 2B1X
Fe in 2B65
Fe in 2B5H
Fe in 2B4Z
Fe in 2B3X
Fe in 2B11
Fe in 2B12
Fe in 2B10