Copper »
PDB 3x2q-4b5q »
3zx1 »
Copper in PDB 3zx1: Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase
Protein crystallography data
The structure of Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase, PDB code: 3zx1
was solved by
C.S.Silva,
P.Durao,
A.Fillat,
P.F.Lindley,
L.O.Martins,
I.Bento,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 49.51 / 1.95 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.810, 94.860, 50.380, 90.00, 100.63, 90.00 |
| R / Rfree (%) | 16.02 / 20.714 |
Copper Binding Sites:
The binding sites of Copper atom in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase
(pdb code 3zx1). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 4 binding sites of Copper where determined in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase, PDB code: 3zx1:
Jump to Copper binding site number: 1; 2; 3; 4;
In total 4 binding sites of Copper where determined in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase, PDB code: 3zx1:
Jump to Copper binding site number: 1; 2; 3; 4;
Copper binding site 1 out of 4 in 3zx1
Go back to
Copper binding site 1 out
of 4 in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase
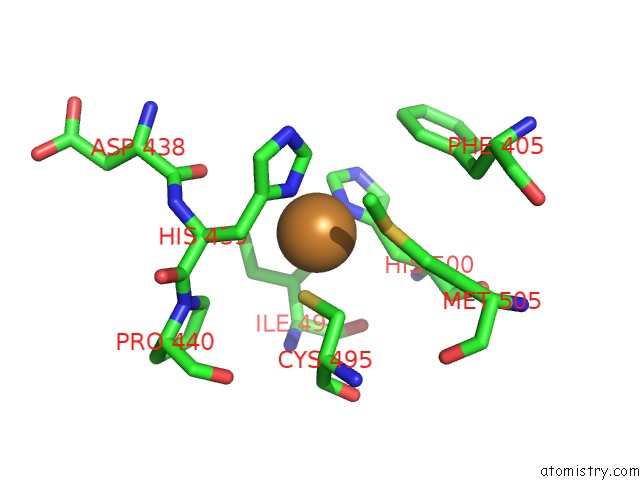
Mono view
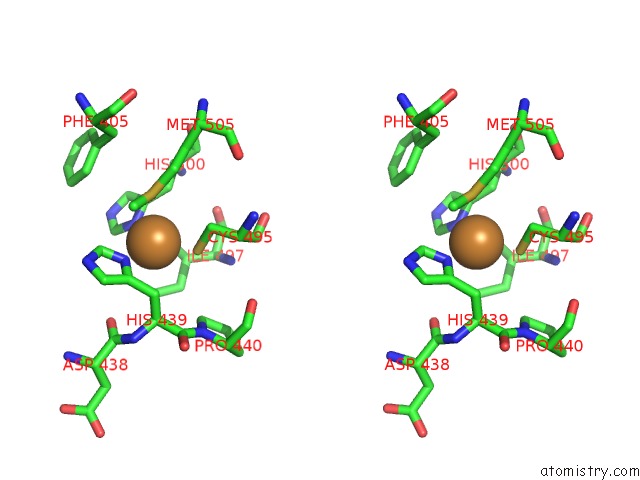
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase within 5.0Å range:
|
Copper binding site 2 out of 4 in 3zx1
Go back to
Copper binding site 2 out
of 4 in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase
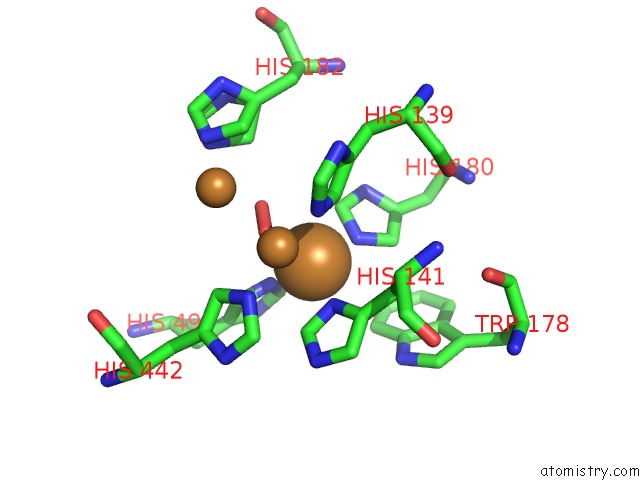
Mono view
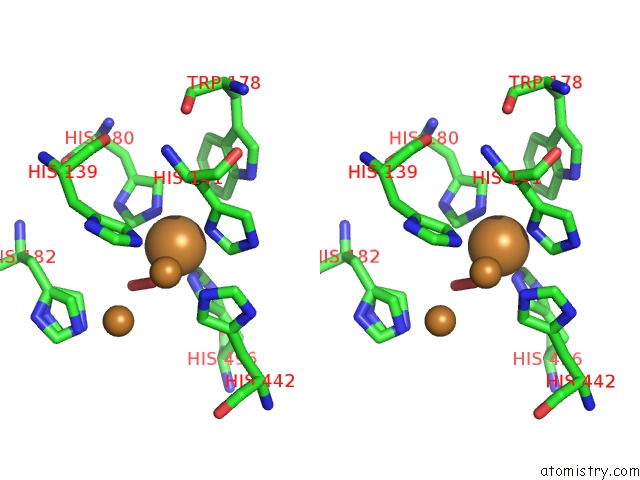
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase within 5.0Å range:
|
Copper binding site 3 out of 4 in 3zx1
Go back to
Copper binding site 3 out
of 4 in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase
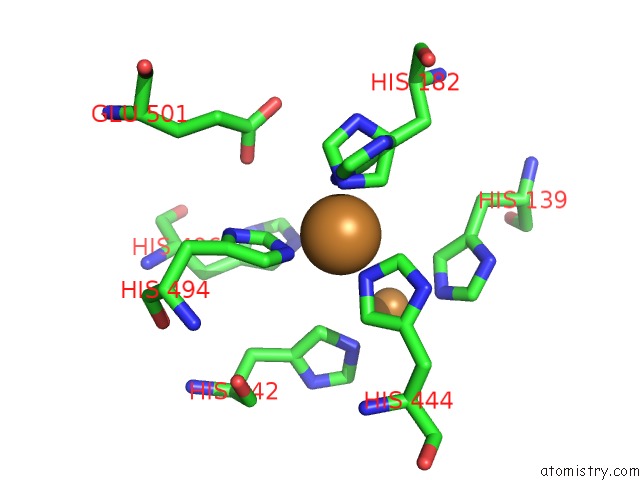
Mono view
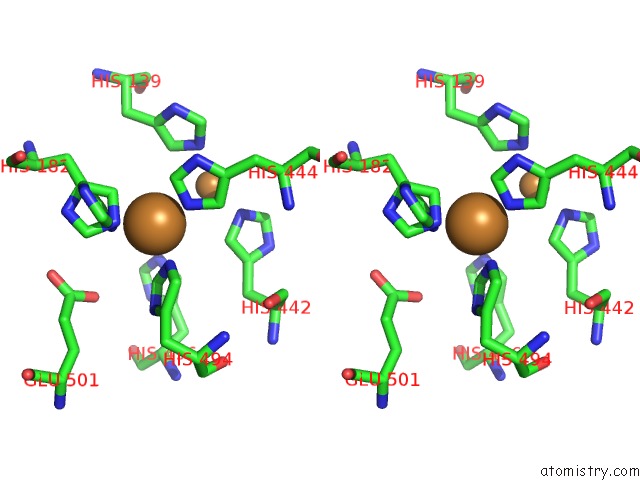
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase within 5.0Å range:
|
Copper binding site 4 out of 4 in 3zx1
Go back to
Copper binding site 4 out
of 4 in the Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase
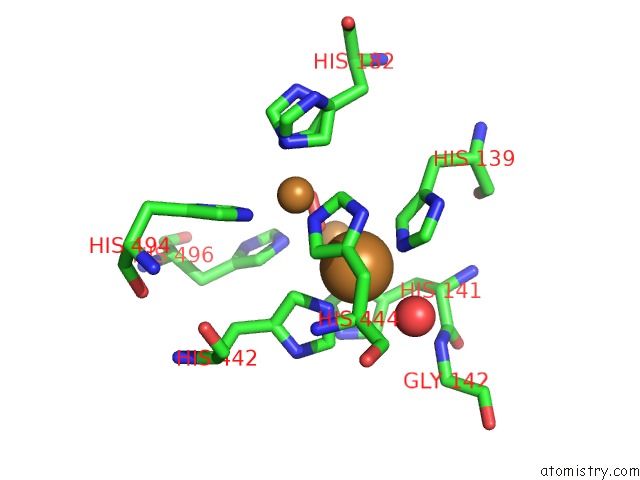
Mono view
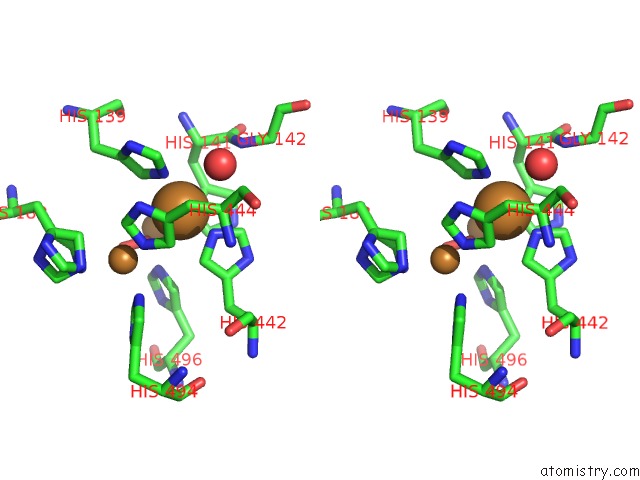
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Multicopper Oxidase From Campylobacter Jejuni: A Metallo-Oxidase within 5.0Å range:
|
Reference:
C.S.Silva,
P.Durao,
A.Fillat,
P.F.Lindley,
L.O.Martins,
I.Bento.
Crystal Structure of the Multicopper Oxidase From the Pathogenic Bacterium Campylobacter Jejuni CGUG11284: Characterization of A Metallo-Oxidase. Metallomics V. 4 37 2012.
ISSN: ISSN 1756-5901
PubMed: 22127520
DOI: 10.1039/C1MT00156F
Page generated: Wed Jul 31 02:31:00 2024
ISSN: ISSN 1756-5901
PubMed: 22127520
DOI: 10.1039/C1MT00156F
Last articles
Cl in 3GXGCl in 3GW6
Cl in 3GWU
Cl in 3GUP
Cl in 3GW5
Cl in 3GR6
Cl in 3GUN
Cl in 3GUO
Cl in 3GUM
Cl in 3GUL