Copper »
PDB 2z7y-3aws »
3ag4 »
Copper in PDB 3ag4: Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
Enzymatic activity of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
All present enzymatic activity of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K:
1.9.3.1;
1.9.3.1;
Protein crystallography data
The structure of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K, PDB code: 3ag4
was solved by
K.Muramoto,
K.Ohta,
K.Shinzawa-Itoh,
K.Kanda,
M.Taniguchi,
H.Nabekura,
E.Yamashita,
T.Tsukihara,
S.Yoshikawa,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 2.05 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 183.364, 206.648, 178.137, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.6 / 21.9 |
Other elements in 3ag4:
The structure of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K also contains other interesting chemical elements:
| Magnesium | (Mg) | 2 atoms |
| Zinc | (Zn) | 2 atoms |
| Iron | (Fe) | 4 atoms |
| Sodium | (Na) | 2 atoms |
Copper Binding Sites:
The binding sites of Copper atom in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
(pdb code 3ag4). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 6 binding sites of Copper where determined in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K, PDB code: 3ag4:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Copper where determined in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K, PDB code: 3ag4:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
Copper binding site 1 out of 6 in 3ag4
Go back to
Copper binding site 1 out
of 6 in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
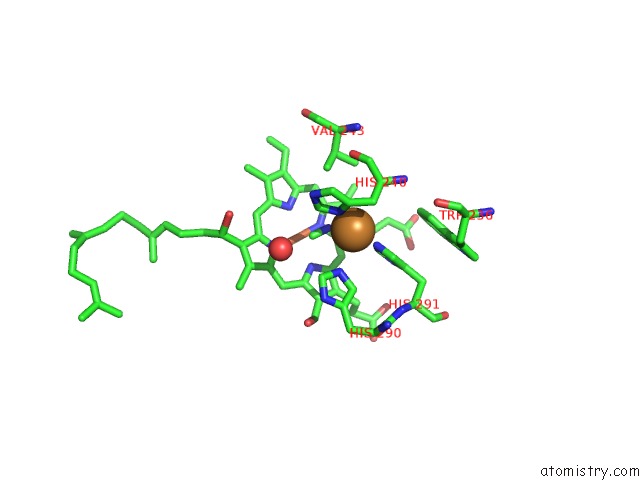
Mono view
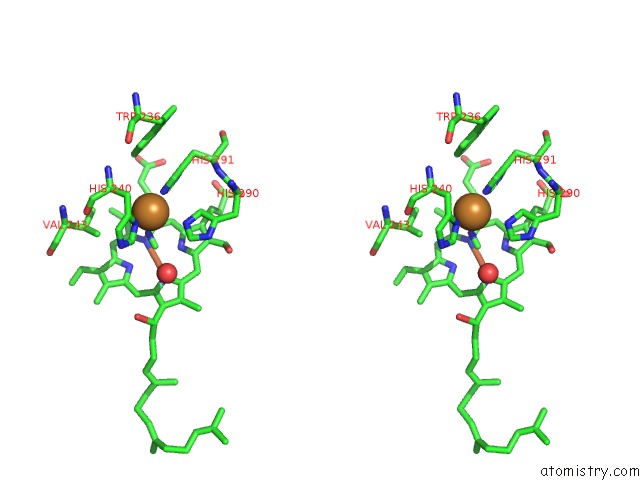
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K within 5.0Å range:
|
Copper binding site 2 out of 6 in 3ag4
Go back to
Copper binding site 2 out
of 6 in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
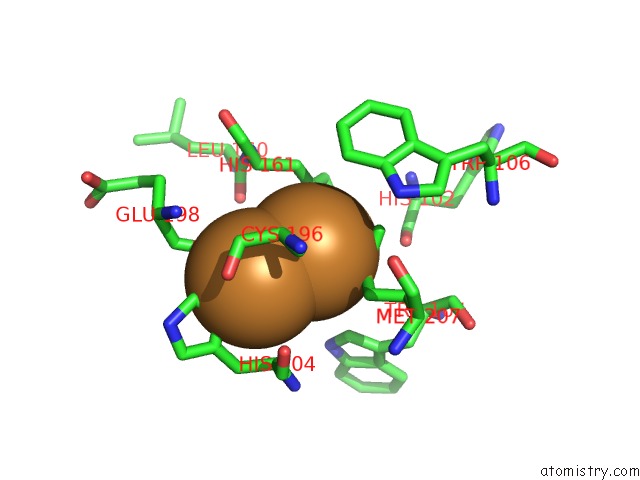
Mono view
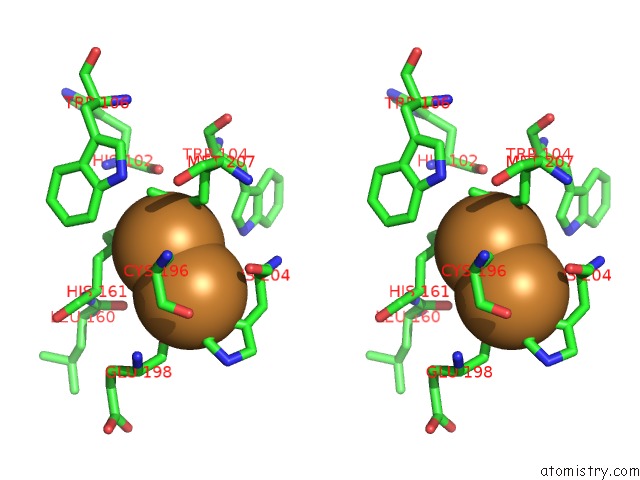
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K within 5.0Å range:
|
Copper binding site 3 out of 6 in 3ag4
Go back to
Copper binding site 3 out
of 6 in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
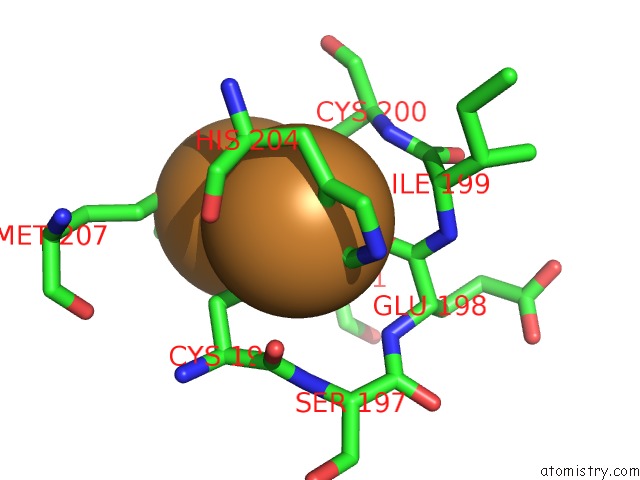
Mono view
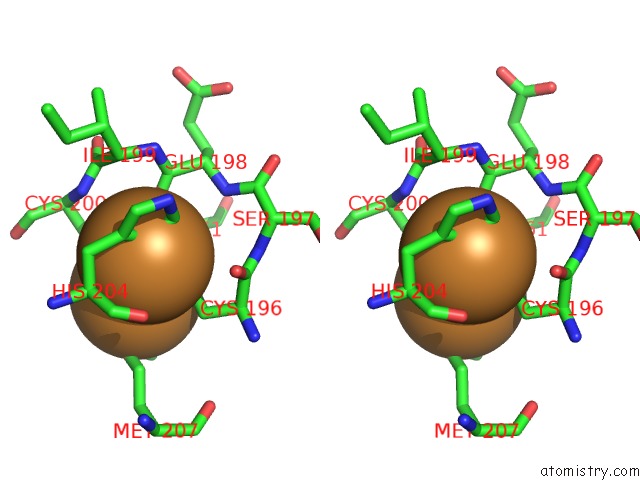
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K within 5.0Å range:
|
Copper binding site 4 out of 6 in 3ag4
Go back to
Copper binding site 4 out
of 6 in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
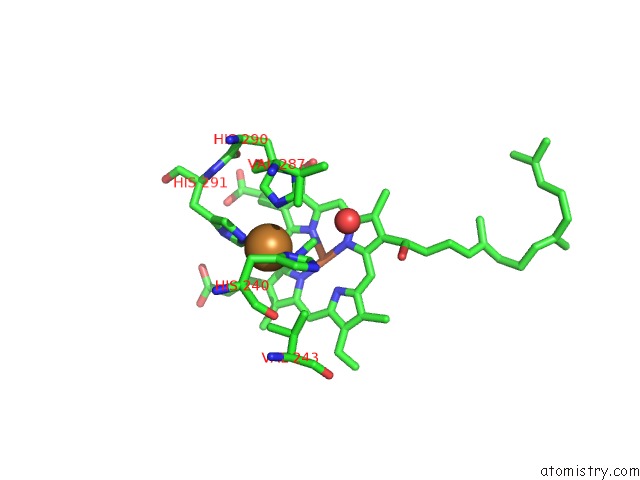
Mono view
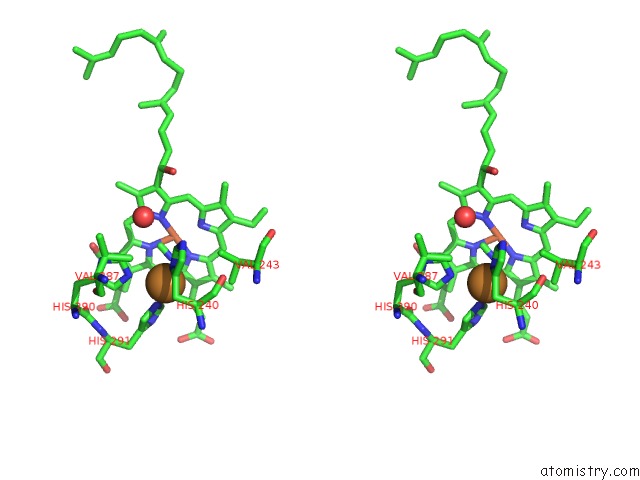
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K within 5.0Å range:
|
Copper binding site 5 out of 6 in 3ag4
Go back to
Copper binding site 5 out
of 6 in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
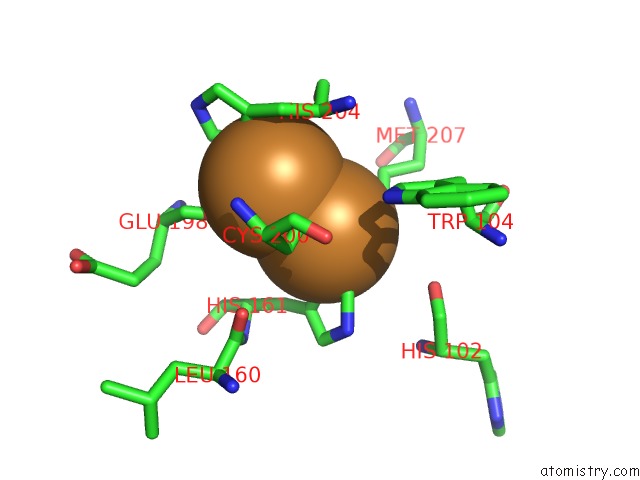
Mono view
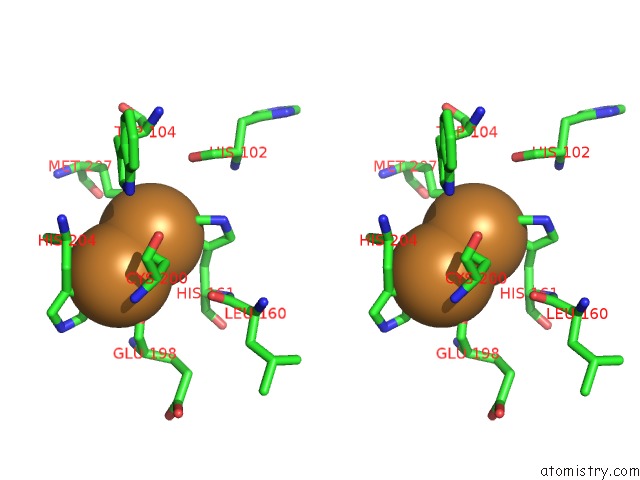
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K within 5.0Å range:
|
Copper binding site 6 out of 6 in 3ag4
Go back to
Copper binding site 6 out
of 6 in the Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K
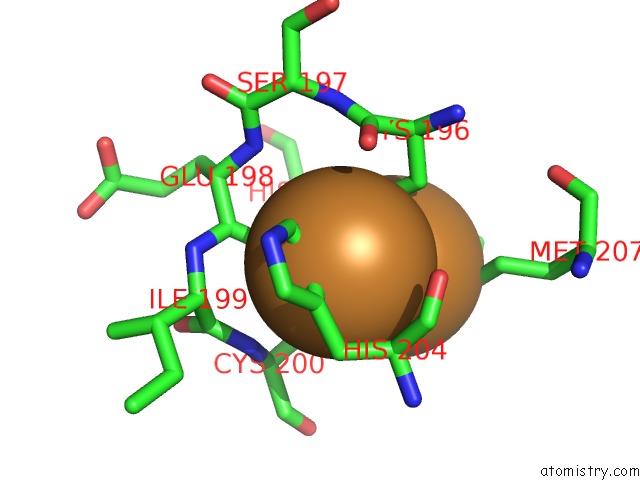
Mono view
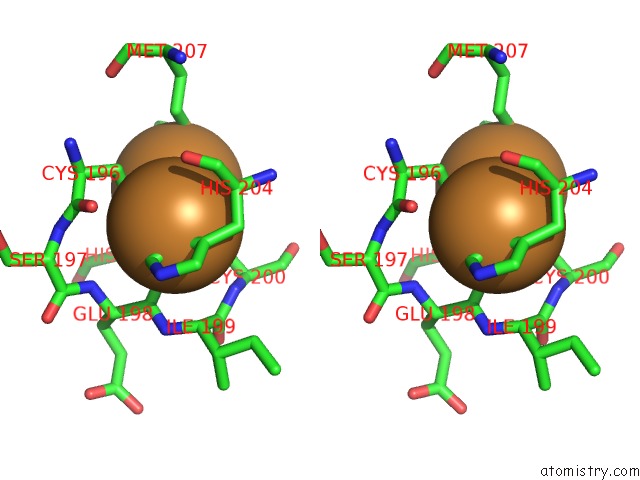
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Bovine Heart Cytochrome C Oxidase in the Cyanide Ion-Bound Fully Reduced State at 100 K within 5.0Å range:
|
Reference:
K.Muramoto,
K.Ohta,
K.Shinzawa-Itoh,
K.Kanda,
M.Taniguchi,
H.Nabekura,
E.Yamashita,
T.Tsukihara,
S.Yoshikawa.
Bovine Cytochrome C Oxidase Structures Enable O2 Reduction with Minimization of Reactive Oxygens and Provide A Proton-Pumping Gate Proc.Natl.Acad.Sci.Usa V. 107 7740 2010.
ISSN: ISSN 0027-8424
PubMed: 20385840
DOI: 10.1073/PNAS.0910410107
Page generated: Mon Jul 14 01:53:28 2025
ISSN: ISSN 0027-8424
PubMed: 20385840
DOI: 10.1073/PNAS.0910410107
Last articles
F in 4H3JF in 4GXJ
F in 4GVH
F in 4GU9
F in 4GSI
F in 4GPT
F in 4GTO
F in 4GS9
F in 4GQ4
F in 4GPB