Copper »
PDB 2xv2-2z7w »
2xxg »
Copper in PDB 2xxg: Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans
Protein crystallography data
The structure of Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans, PDB code: 2xxg
was solved by
M.A.Hough,
R.R.Eady,
S.S.Hasnain,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.16 / 1.60 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 89.552, 89.552, 287.678, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 16.75 / 21.074 |
Other elements in 2xxg:
The structure of Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans also contains other interesting chemical elements:
| Zinc | (Zn) | 4 atoms |
Copper Binding Sites:
The binding sites of Copper atom in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans
(pdb code 2xxg). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 4 binding sites of Copper where determined in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans, PDB code: 2xxg:
Jump to Copper binding site number: 1; 2; 3; 4;
In total 4 binding sites of Copper where determined in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans, PDB code: 2xxg:
Jump to Copper binding site number: 1; 2; 3; 4;
Copper binding site 1 out of 4 in 2xxg
Go back to
Copper binding site 1 out
of 4 in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans

Mono view
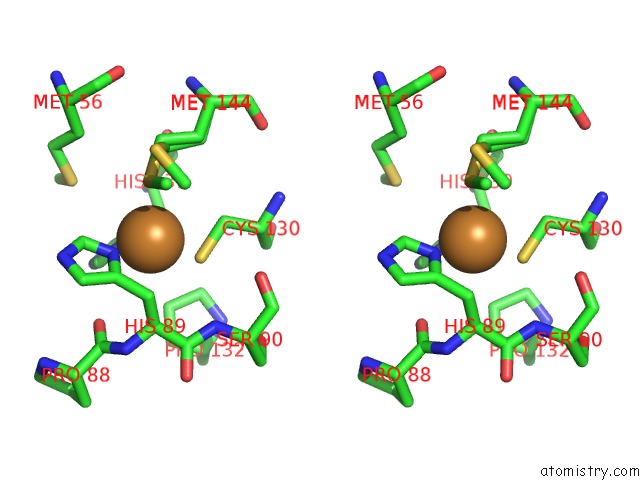
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans within 5.0Å range:
|
Copper binding site 2 out of 4 in 2xxg
Go back to
Copper binding site 2 out
of 4 in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans
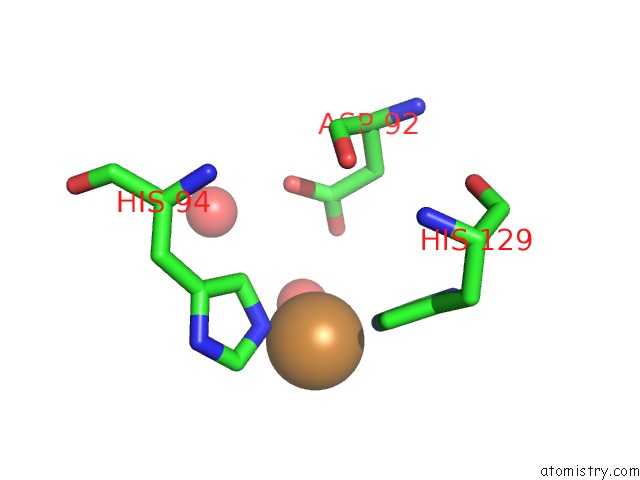
Mono view
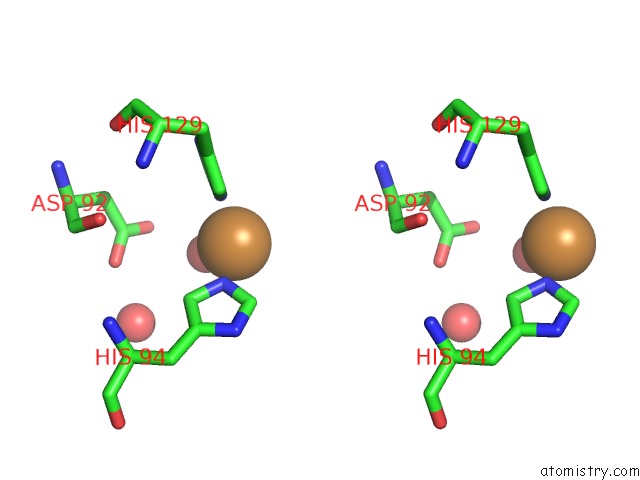
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans within 5.0Å range:
|
Copper binding site 3 out of 4 in 2xxg
Go back to
Copper binding site 3 out
of 4 in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans
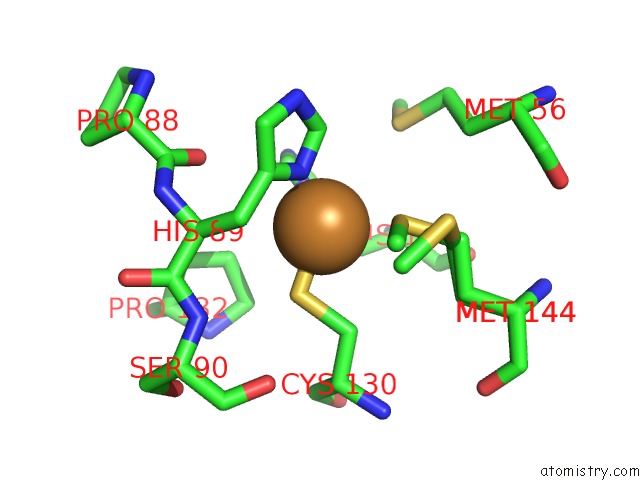
Mono view
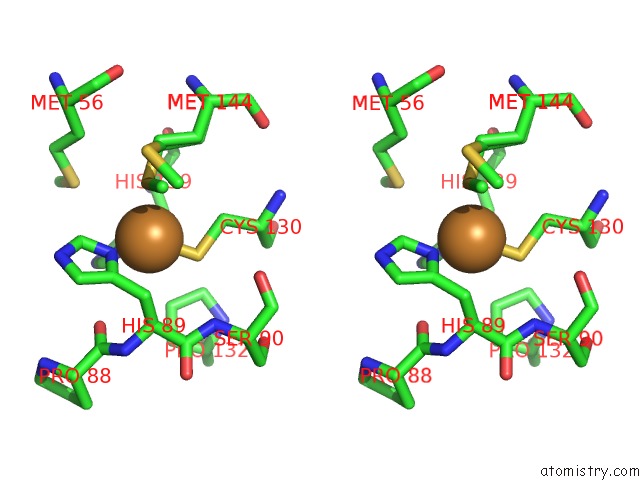
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans within 5.0Å range:
|
Copper binding site 4 out of 4 in 2xxg
Go back to
Copper binding site 4 out
of 4 in the Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Structure of the N90S Mutant of Nitrite Reductase From Alcaligenes Xylosoxidans within 5.0Å range:
|
Reference:
M.A.Hough,
R.R.Eady,
S.S.Hasnain.
Identification of the Proton Channel to the Active Site Type 2 Cu Centre of Nitrite Reductase: Structural and Enzymatic Properties of the HIS254PHE and ASN90SER Mutants Biochemistry V. 47 13547 2008.
ISSN: ISSN 0006-2960
PubMed: 19053252
DOI: 10.1021/BI801369Y
Page generated: Mon Jul 14 01:37:19 2025
ISSN: ISSN 0006-2960
PubMed: 19053252
DOI: 10.1021/BI801369Y
Last articles
F in 7MSOF in 7MSD
F in 7MT4
F in 7MSB
F in 7MSK
F in 7MS5
F in 7MRD
F in 7MS6
F in 7MR8
F in 7MRC