Copper »
PDB 2vr7-2xv0 »
2x88 »
Copper in PDB 2x88: Crystal Structure of Holocota
Protein crystallography data
The structure of Crystal Structure of Holocota, PDB code: 2x88
was solved by
I.Bento,
C.S.Silva,
Z.Chen,
L.O.Martins,
P.F.Lindley,
C.M.Soares,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 87.85 / 1.80 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 101.444, 101.444, 137.257, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.9 / 20.6 |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of Holocota
(pdb code 2x88). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 5 binding sites of Copper where determined in the Crystal Structure of Holocota, PDB code: 2x88:
Jump to Copper binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Copper where determined in the Crystal Structure of Holocota, PDB code: 2x88:
Jump to Copper binding site number: 1; 2; 3; 4; 5;
Copper binding site 1 out of 5 in 2x88
Go back to
Copper binding site 1 out
of 5 in the Crystal Structure of Holocota
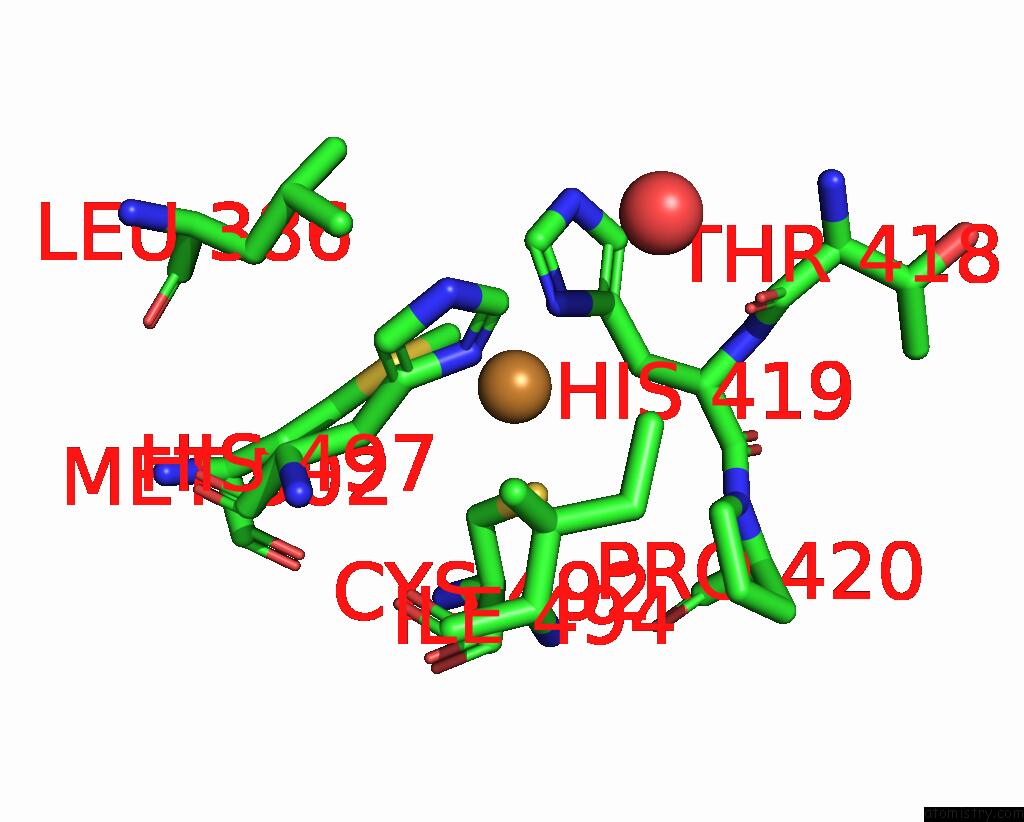
Mono view
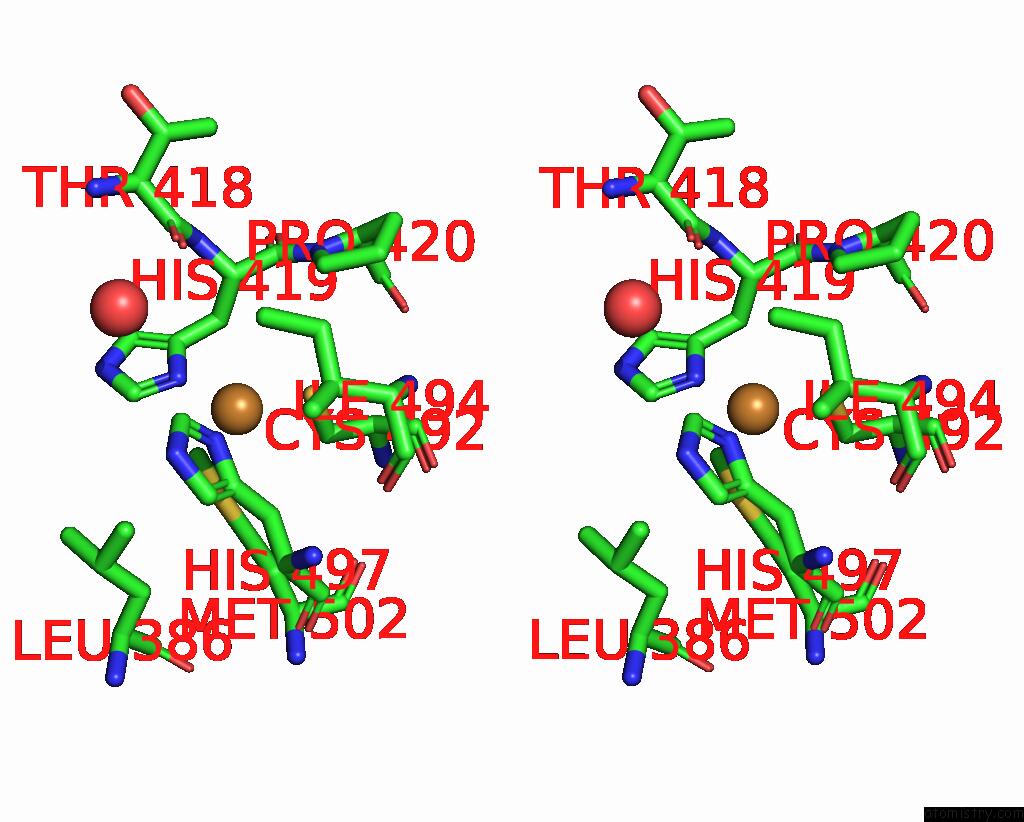
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of Holocota within 5.0Å range:
|
Copper binding site 2 out of 5 in 2x88
Go back to
Copper binding site 2 out
of 5 in the Crystal Structure of Holocota
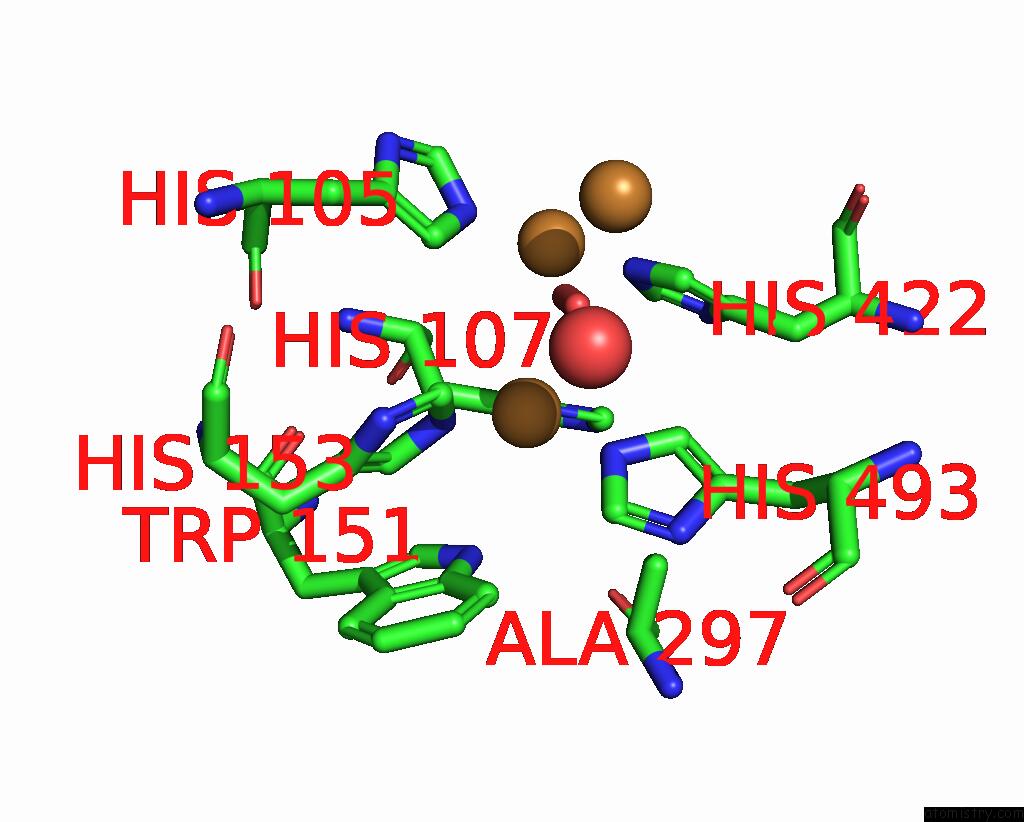
Mono view
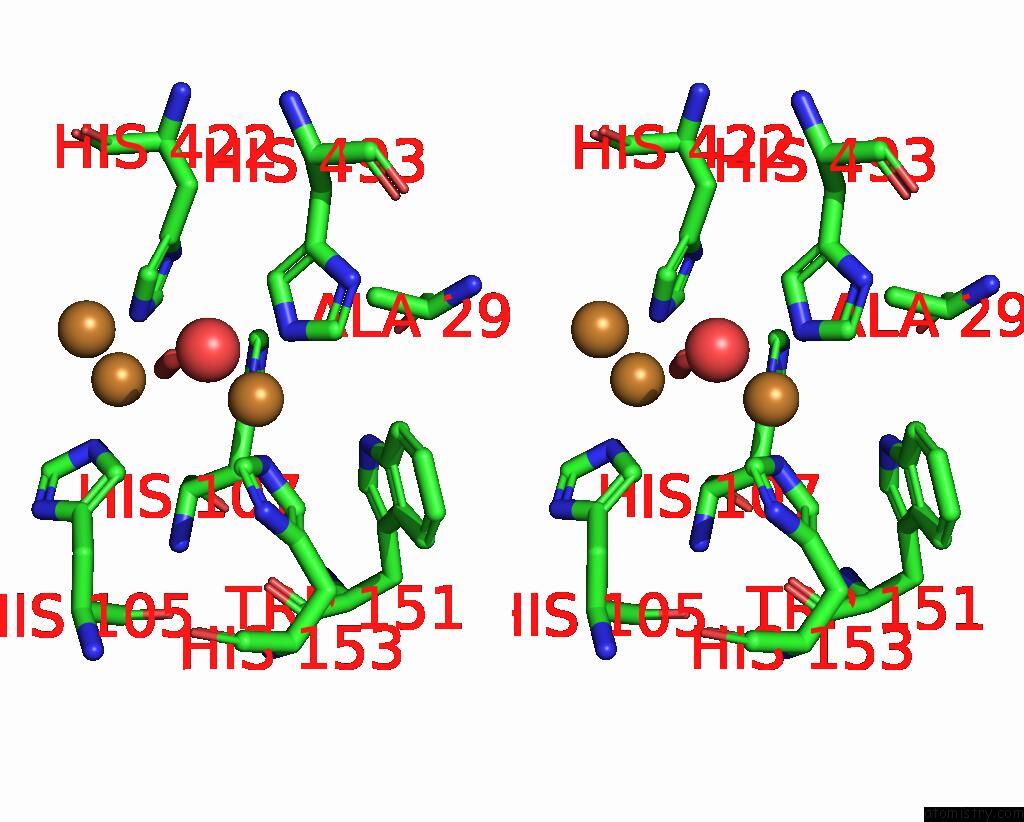
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Crystal Structure of Holocota within 5.0Å range:
|
Copper binding site 3 out of 5 in 2x88
Go back to
Copper binding site 3 out
of 5 in the Crystal Structure of Holocota
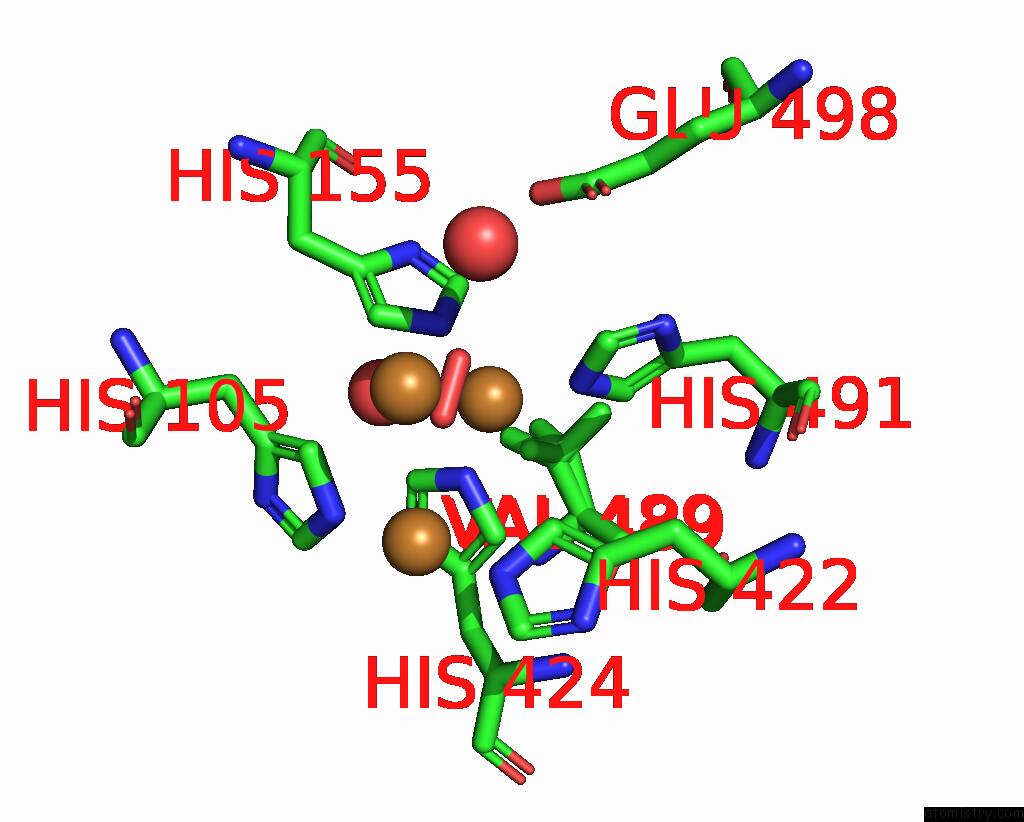
Mono view
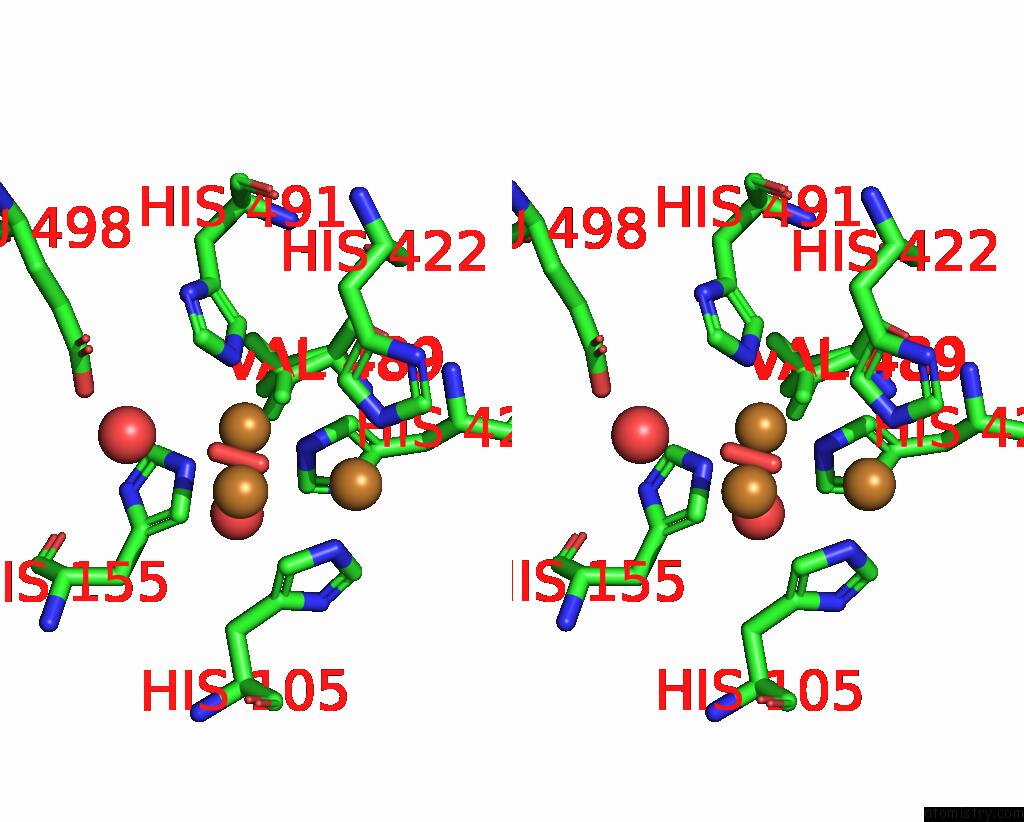
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Crystal Structure of Holocota within 5.0Å range:
|
Copper binding site 4 out of 5 in 2x88
Go back to
Copper binding site 4 out
of 5 in the Crystal Structure of Holocota
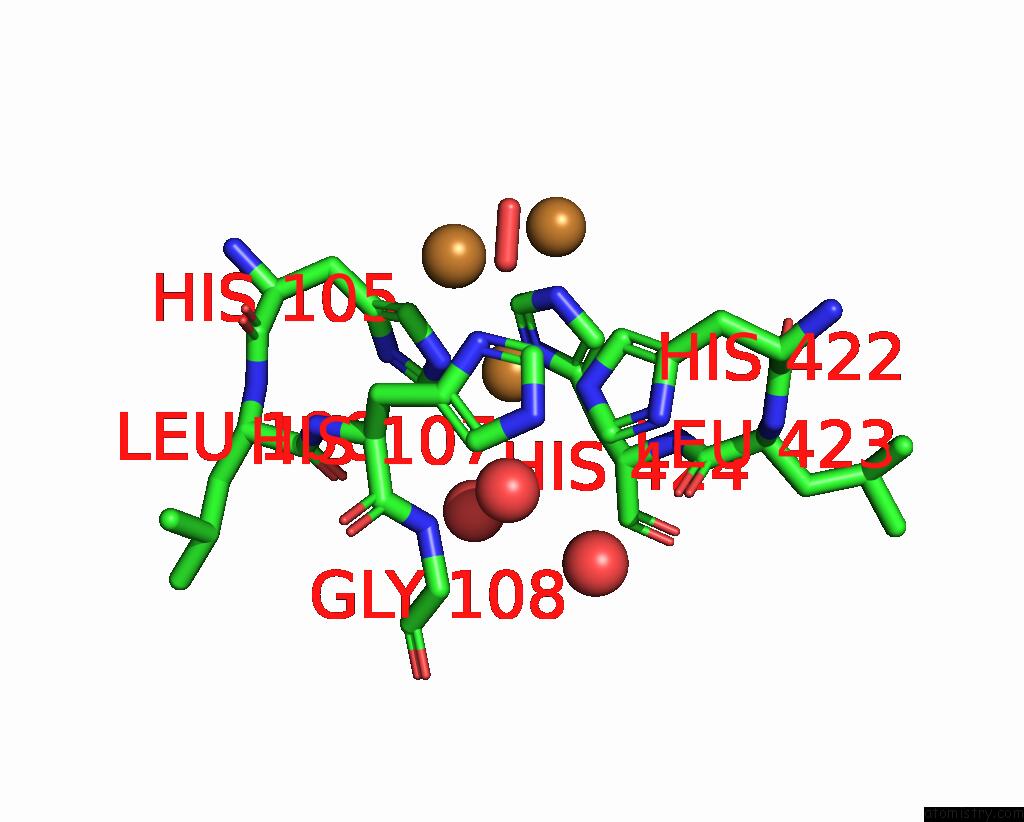
Mono view
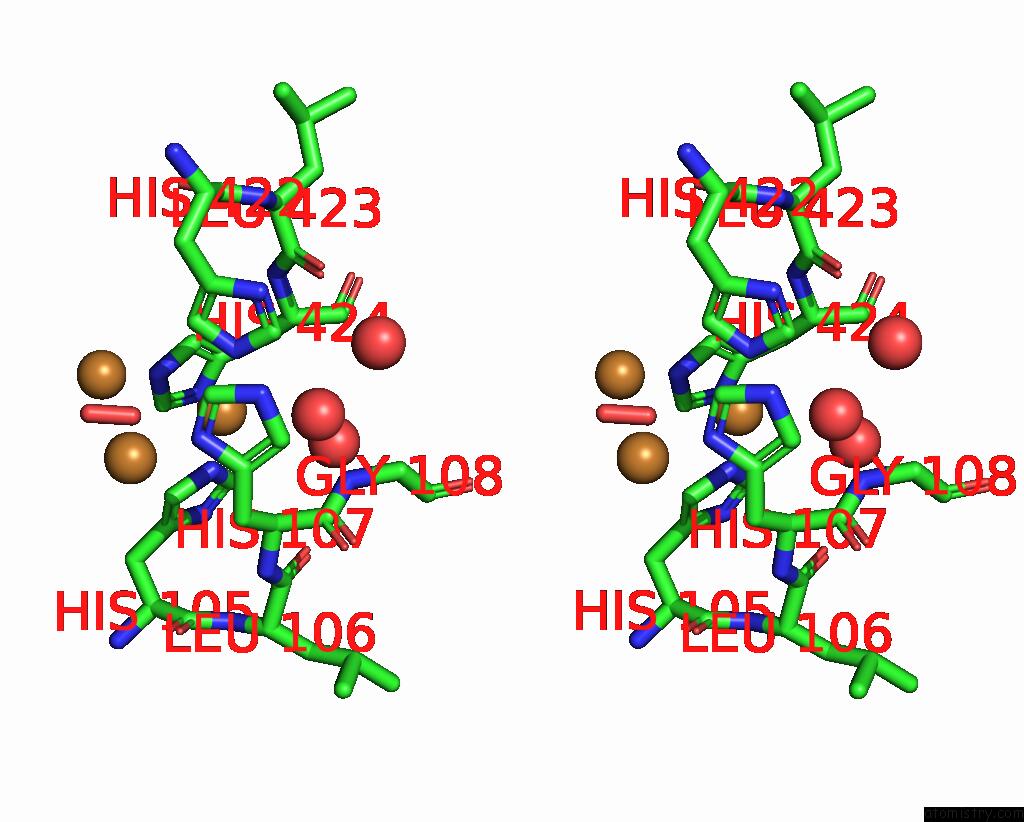
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Crystal Structure of Holocota within 5.0Å range:
|
Copper binding site 5 out of 5 in 2x88
Go back to
Copper binding site 5 out
of 5 in the Crystal Structure of Holocota
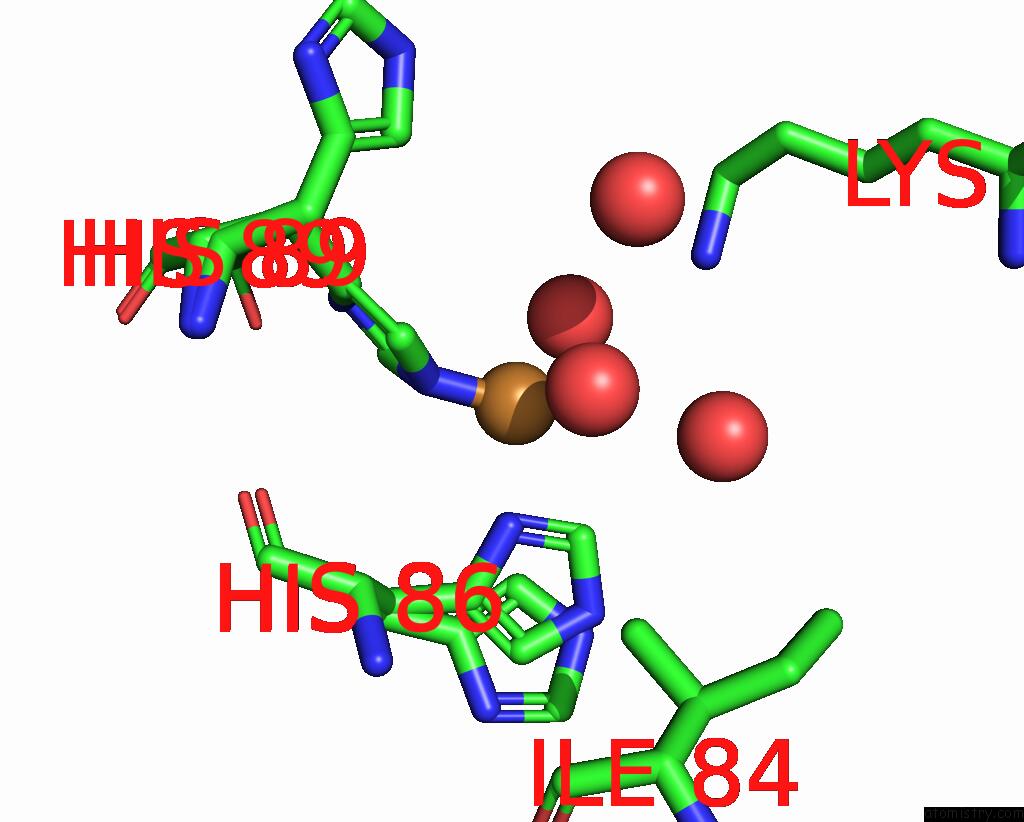
Mono view
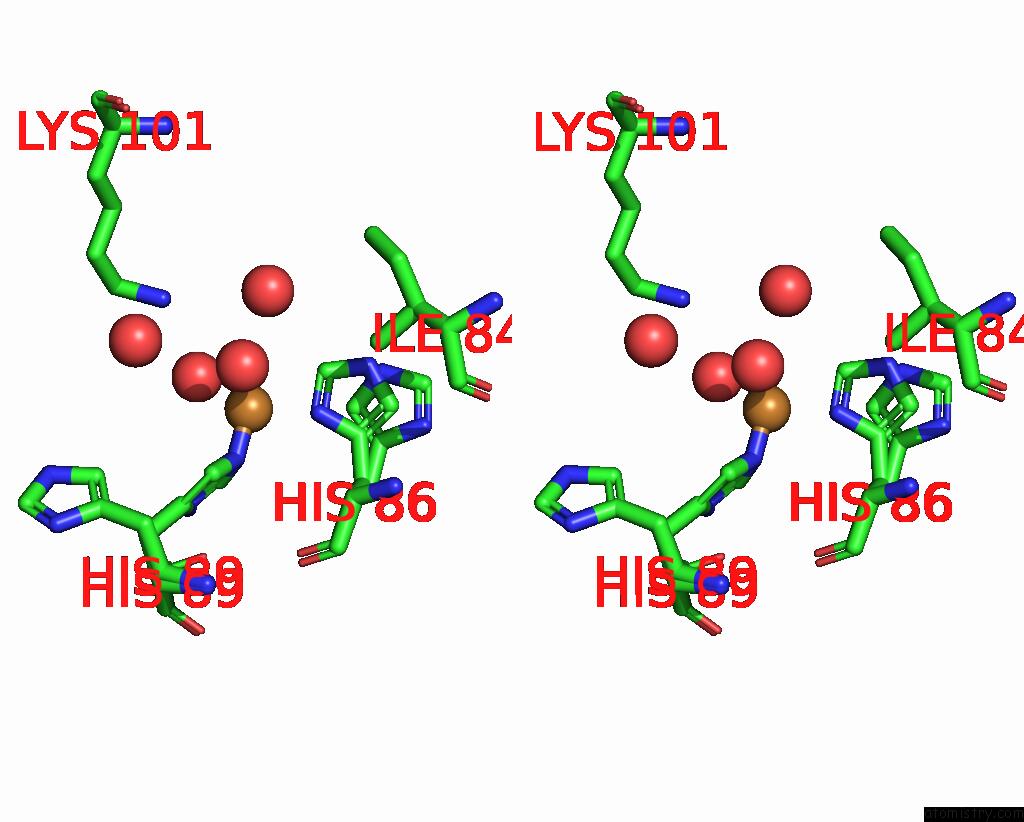
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Crystal Structure of Holocota within 5.0Å range:
|
Reference:
I.Bento,
C.S.Silva,
Z.Chen,
L.O.Martins,
P.F.Lindley,
C.M.Soares.
Mechanisms Underlying Dioxygen Reduction in Laccases. Structural and Modelling Studies Focusing on Proton Transfer. Bmc Struct.Biol. V. 10 29 2010.
ISSN: ESSN 1472-6807
PubMed: 20822511
DOI: 10.1186/1472-6807-10-28
Page generated: Mon Jul 14 01:30:29 2025
ISSN: ESSN 1472-6807
PubMed: 20822511
DOI: 10.1186/1472-6807-10-28
Last articles
F in 7MLDF in 7MKX
F in 7MGK
F in 7MGJ
F in 7MHD
F in 7MFH
F in 7MHC
F in 7MGE
F in 7MFD
F in 7MEW