Copper »
PDB 2vr7-2xv0 »
2wsd »
Copper in PDB 2wsd: Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
Enzymatic activity of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
All present enzymatic activity of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant:
1.10.3.2;
1.10.3.2;
Protein crystallography data
The structure of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant, PDB code: 2wsd
was solved by
C.S.Silva,
P.Durao,
Z.Chen,
C.M.Soares,
M.M.Pereira,
S.Todorovic,
P.Hildebrandt,
L.O.Martins,
P.F.Lindley,
I.Bento,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.99 / 1.60 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 101.868, 101.868, 137.037, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 17.7 / 19 |
Copper Binding Sites:
The binding sites of Copper atom in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
(pdb code 2wsd). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 4 binding sites of Copper where determined in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant, PDB code: 2wsd:
Jump to Copper binding site number: 1; 2; 3; 4;
In total 4 binding sites of Copper where determined in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant, PDB code: 2wsd:
Jump to Copper binding site number: 1; 2; 3; 4;
Copper binding site 1 out of 4 in 2wsd
Go back to
Copper binding site 1 out
of 4 in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
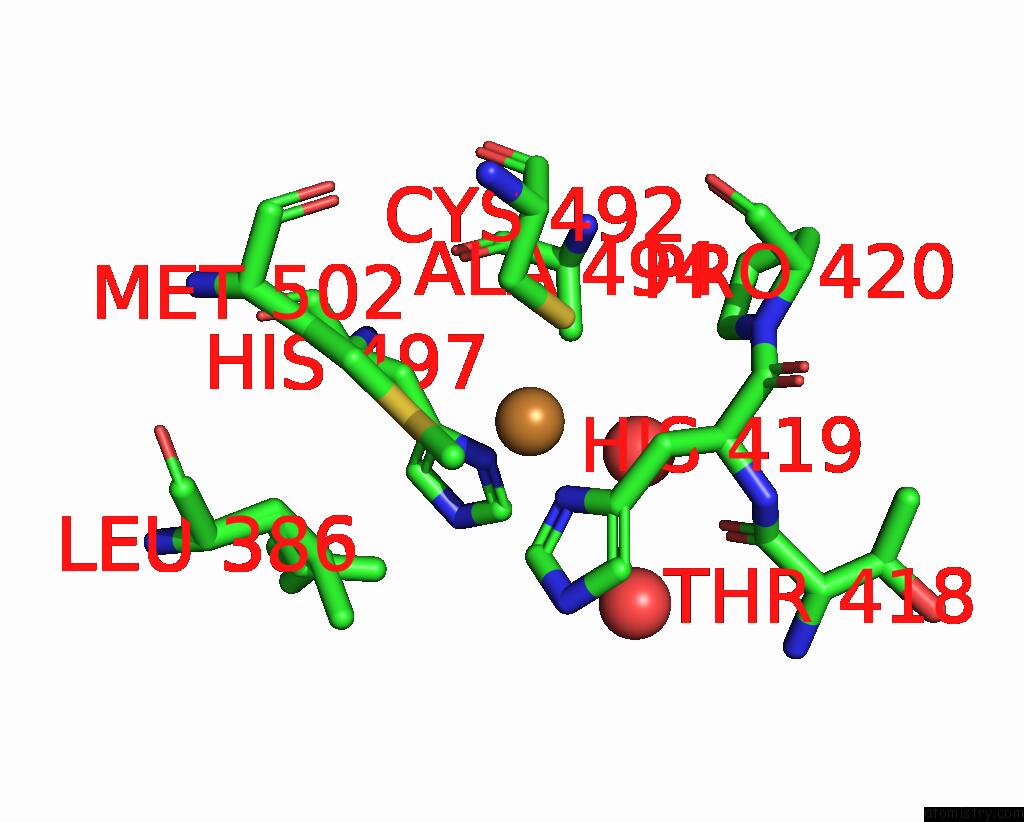
Mono view
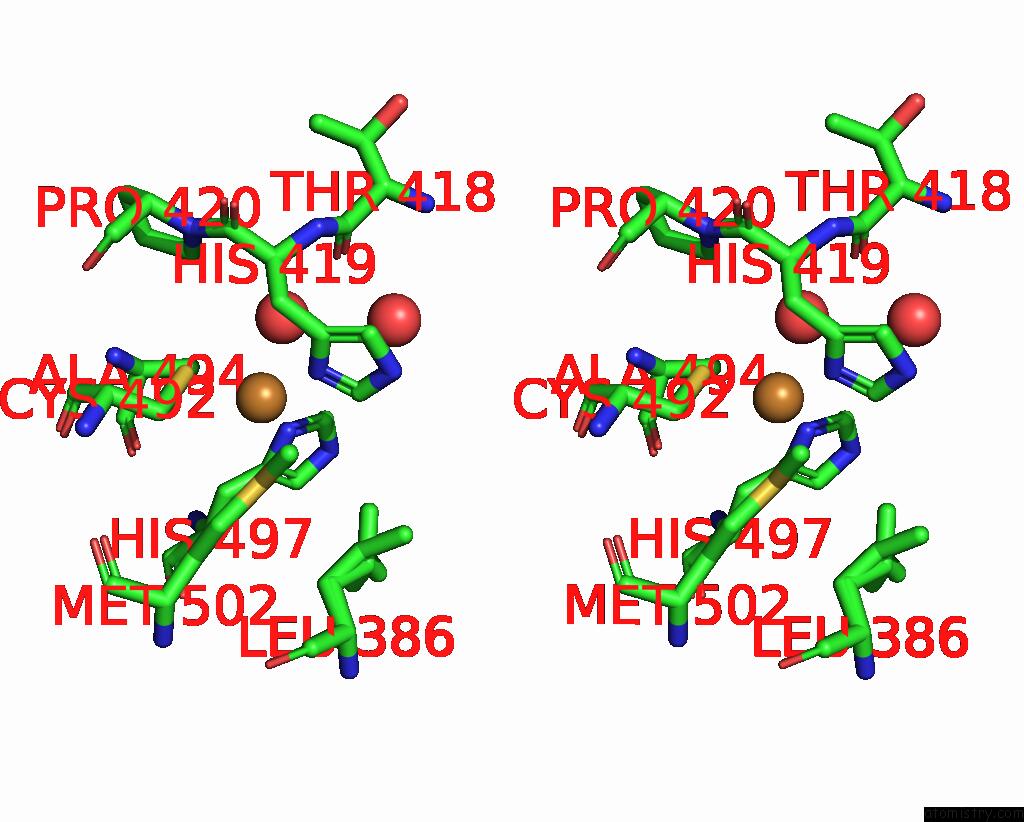
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant within 5.0Å range:
|
Copper binding site 2 out of 4 in 2wsd
Go back to
Copper binding site 2 out
of 4 in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
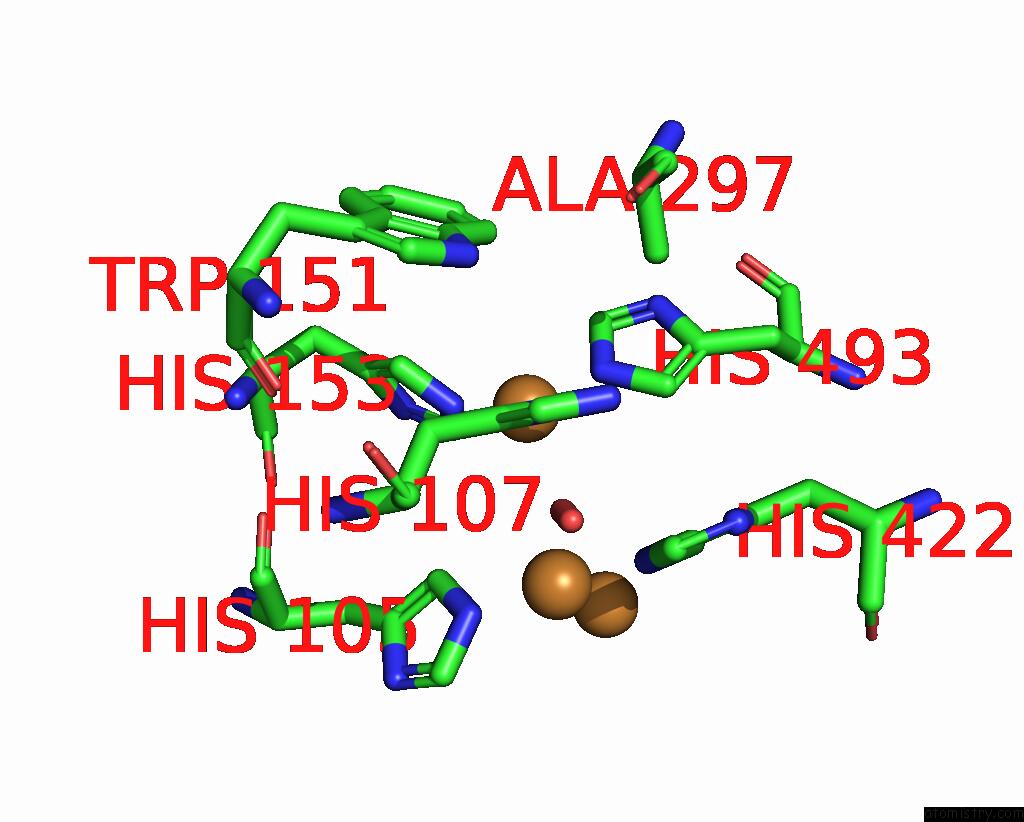
Mono view
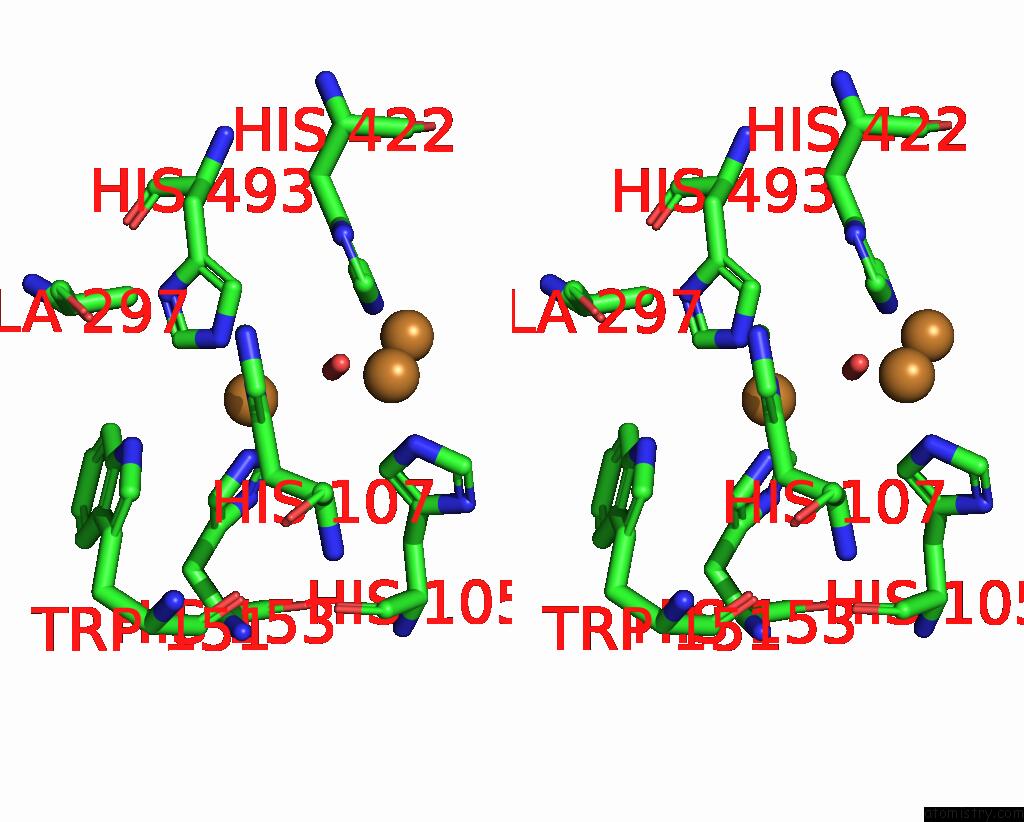
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant within 5.0Å range:
|
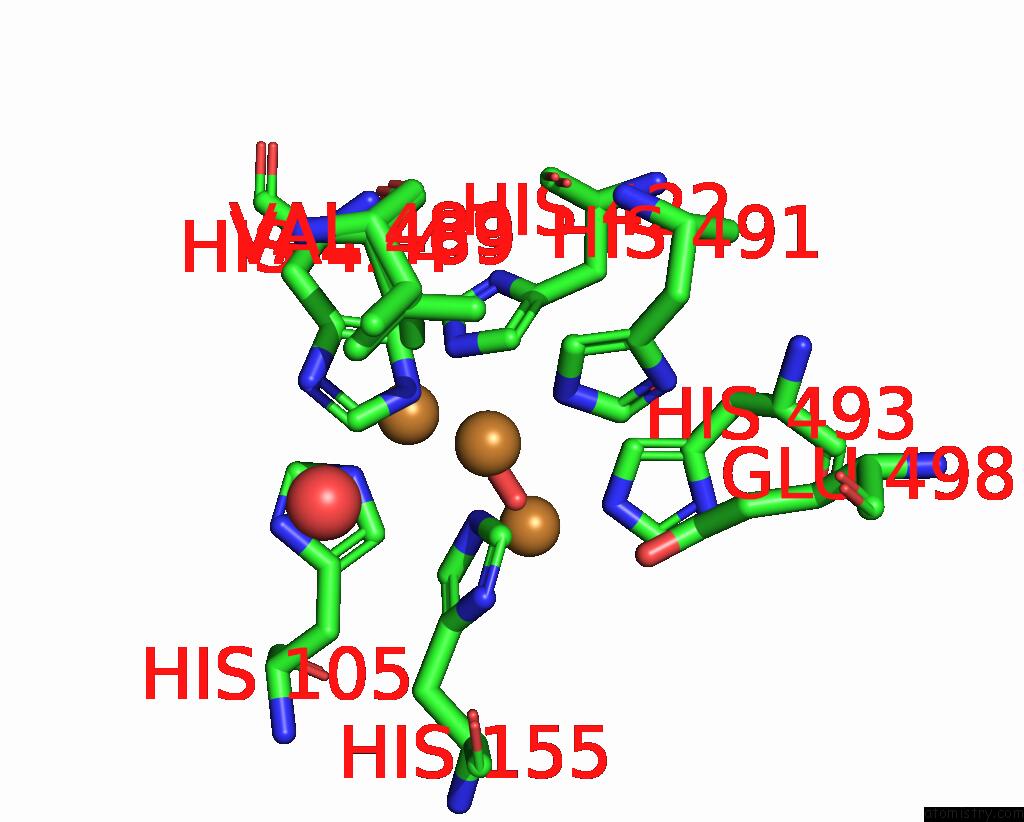
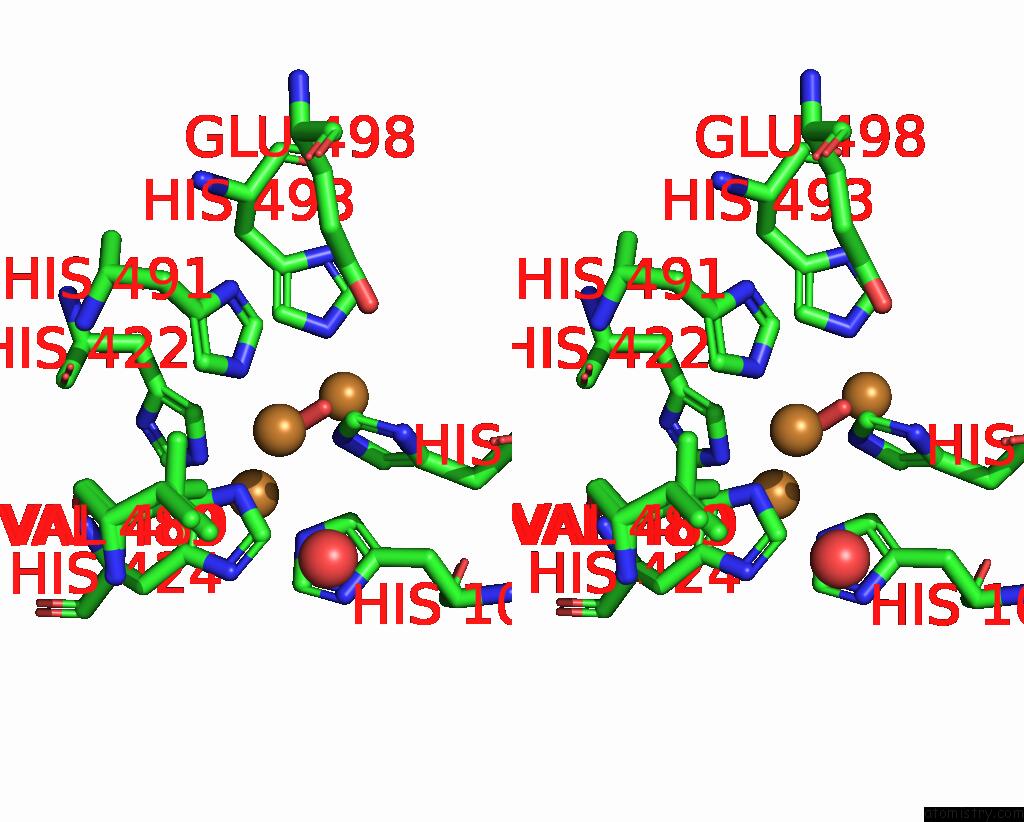
Copper binding site 3 out of 4 in 2wsd
Go back to
Copper binding site 3 out
of 4 in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant within 5.0Å range:
|
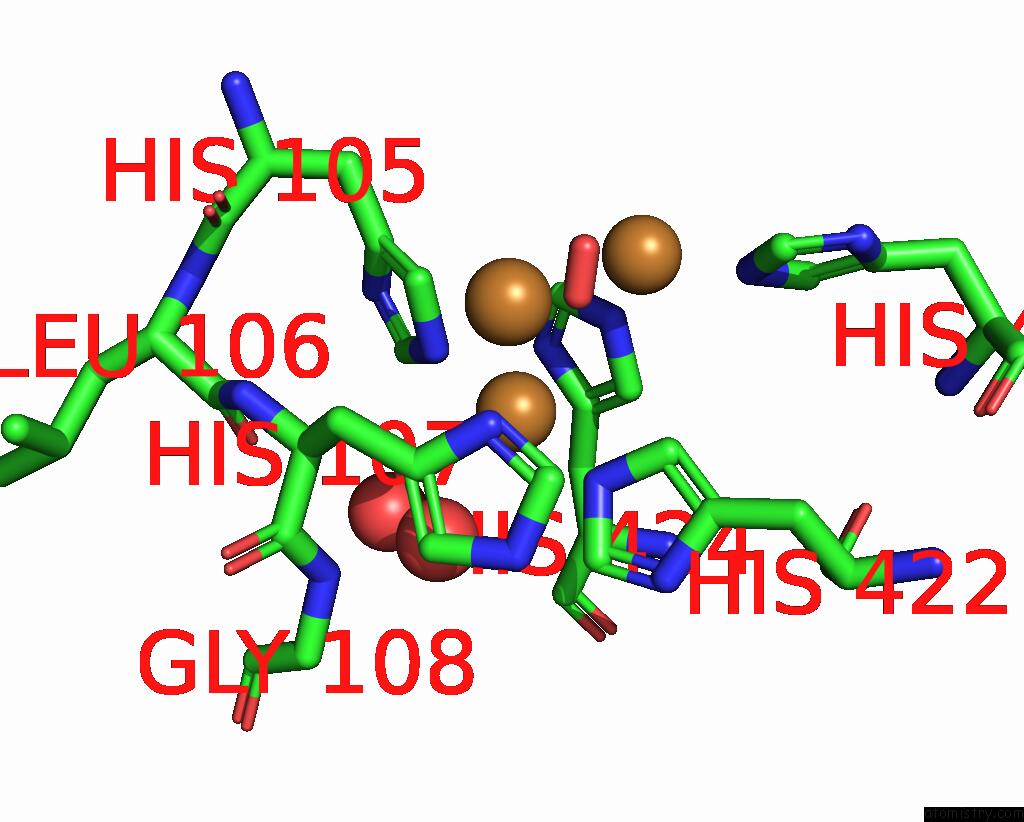
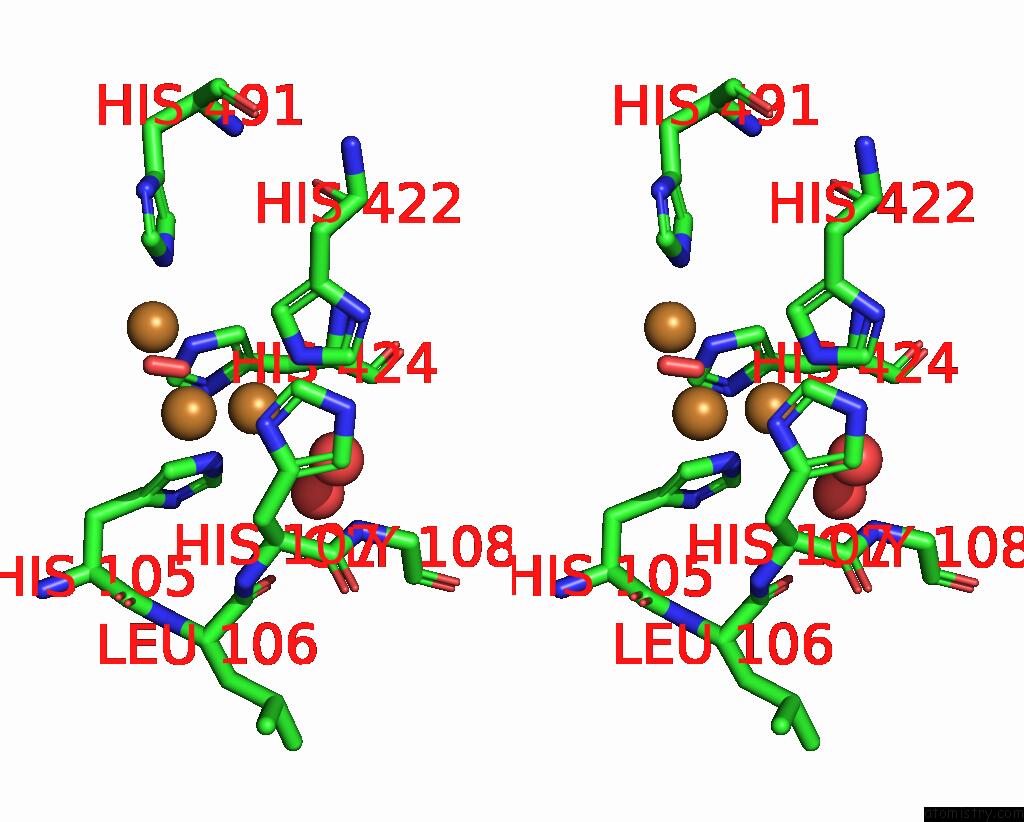
Copper binding site 4 out of 4 in 2wsd
Go back to
Copper binding site 4 out
of 4 in the Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Proximal Mutations at the Type 1 Cu Site of Cota-Laccase: I494A Mutant within 5.0Å range:
|
Reference:
P.Durao,
Z.Chen,
C.S.Silva,
C.M.Soares,
M.M.Pereira,
S.Todorovic,
P.Hildebrandt,
I.Bento,
P.F.Lindley,
L.O.Martins.
Proximal Mutations at the Type 1 Copper Site of Cota Laccase: Spectroscopic, Redox, Kinetic and Structural Characterization of I494A and L386A Mutants. Biochem.J. V. 412 339 2008.
ISSN: ISSN 0264-6021
PubMed: 18307408
DOI: 10.1042/BJ20080166
Page generated: Mon Jul 14 01:29:06 2025
ISSN: ISSN 0264-6021
PubMed: 18307408
DOI: 10.1042/BJ20080166
Last articles
F in 4C73F in 4CAM
F in 4C9X
F in 4C9W
F in 4C6M
F in 4C6L
F in 4C5S
F in 4C5R
F in 4C62
F in 4C61