Copper »
PDB 2pp8-2vr6 »
2qpe »
Copper in PDB 2qpe: An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus
Enzymatic activity of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus
All present enzymatic activity of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus:
1.9.3.1;
1.9.3.1;
Protein crystallography data
The structure of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus, PDB code: 2qpe
was solved by
B.Liu,
V.M.Luna,
Y.Chen,
C.D.Stout,
J.A.Fee,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.99 / 2.90 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.635, 114.635, 148.568, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 23.4 / 30.2 |
Other elements in 2qpe:
The structure of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus also contains other interesting chemical elements:
| Iron | (Fe) | 2 atoms |
Copper Binding Sites:
The binding sites of Copper atom in the An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus
(pdb code 2qpe). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 3 binding sites of Copper where determined in the An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus, PDB code: 2qpe:
Jump to Copper binding site number: 1; 2; 3;
In total 3 binding sites of Copper where determined in the An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus, PDB code: 2qpe:
Jump to Copper binding site number: 1; 2; 3;
Copper binding site 1 out of 3 in 2qpe
Go back to
Copper binding site 1 out
of 3 in the An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus within 5.0Å range:
|
Copper binding site 2 out of 3 in 2qpe
Go back to
Copper binding site 2 out
of 3 in the An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus

Mono view
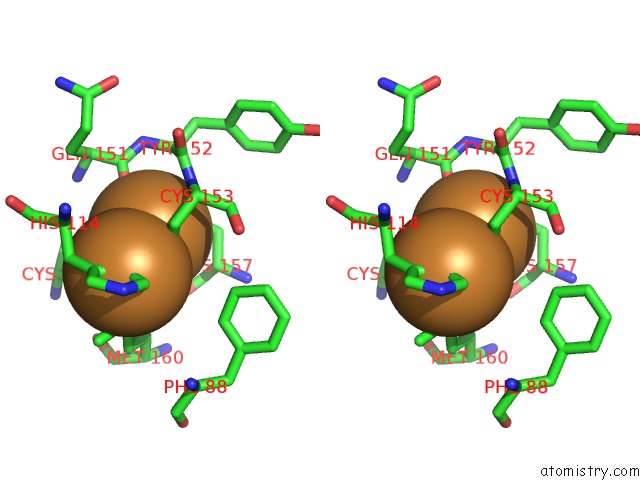
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus within 5.0Å range:
|
Copper binding site 3 out of 3 in 2qpe
Go back to
Copper binding site 3 out
of 3 in the An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus
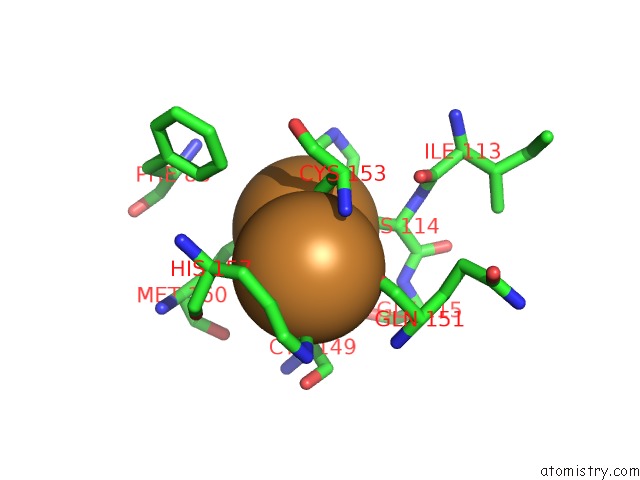
Mono view
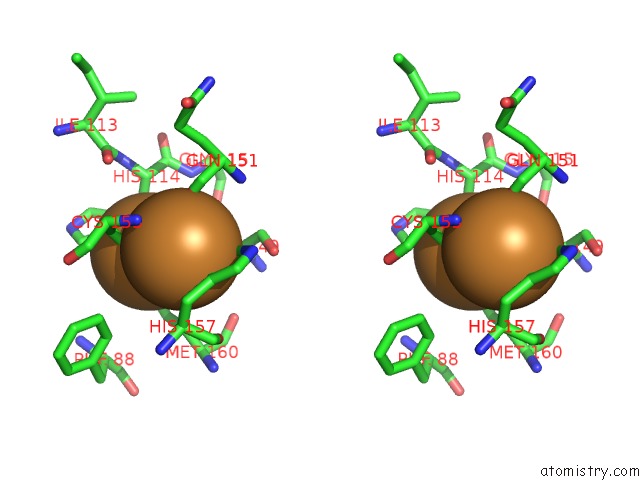
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of An Unexpected Outcome of Surface-Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome BA3 Oxidase From Thermus Thermophilus within 5.0Å range:
|
Reference:
B.Liu,
V.M.Luna,
Y.Chen,
C.D.Stout,
J.A.Fee.
An Unexpected Outcome of Surface Engineering An Integral Membrane Protein: Improved Crystallization of Cytochrome Ba(3) From Thermus Thermophilus. Acta Crystallogr.,Sect.F V. 63 1029 2007.
ISSN: ESSN 1744-3091
PubMed: 18084085
DOI: 10.1107/S1744309107054176
Page generated: Wed Jul 31 00:00:39 2024
ISSN: ESSN 1744-3091
PubMed: 18084085
DOI: 10.1107/S1744309107054176
Last articles
Cl in 3DI6Cl in 3DHP
Cl in 3DH6
Cl in 3DGQ
Cl in 3DH5
Cl in 3DHK
Cl in 3DHH
Cl in 3DCI
Cl in 3DGC
Cl in 3DGO