Copper »
PDB 2pp8-2vr6 »
2pp8 »
Copper in PDB 2pp8: Formate Bound to Oxidized Wild Type Afnir
Enzymatic activity of Formate Bound to Oxidized Wild Type Afnir
All present enzymatic activity of Formate Bound to Oxidized Wild Type Afnir:
1.7.2.1;
1.7.2.1;
Protein crystallography data
The structure of Formate Bound to Oxidized Wild Type Afnir, PDB code: 2pp8
was solved by
E.I.Tocheva,
L.D.Eltis,
M.E.P.Murphy,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 1.50 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 61.334, 102.453, 146.179, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.4 / 20.1 |
Copper Binding Sites:
The binding sites of Copper atom in the Formate Bound to Oxidized Wild Type Afnir
(pdb code 2pp8). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 6 binding sites of Copper where determined in the Formate Bound to Oxidized Wild Type Afnir, PDB code: 2pp8:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Copper where determined in the Formate Bound to Oxidized Wild Type Afnir, PDB code: 2pp8:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6;
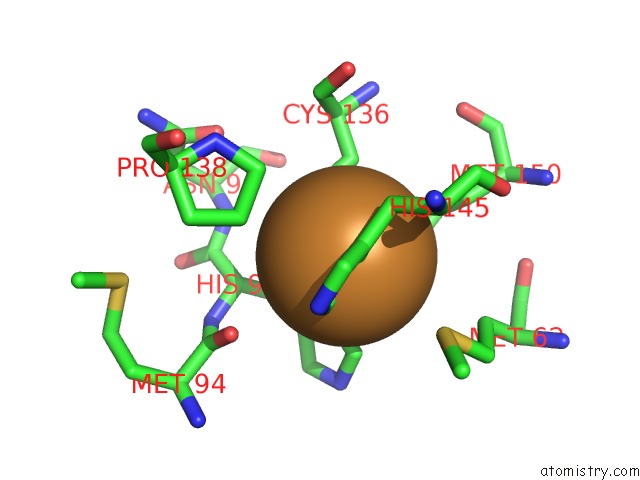
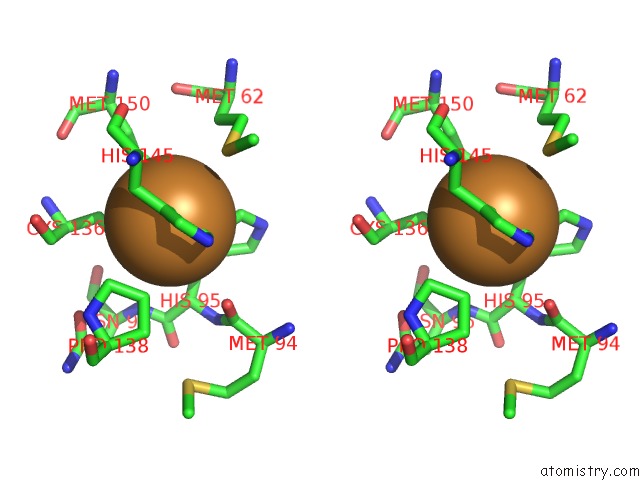
Copper binding site 1 out of 6 in 2pp8
Go back to
Copper binding site 1 out
of 6 in the Formate Bound to Oxidized Wild Type Afnir
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Formate Bound to Oxidized Wild Type Afnir within 5.0Å range:
|
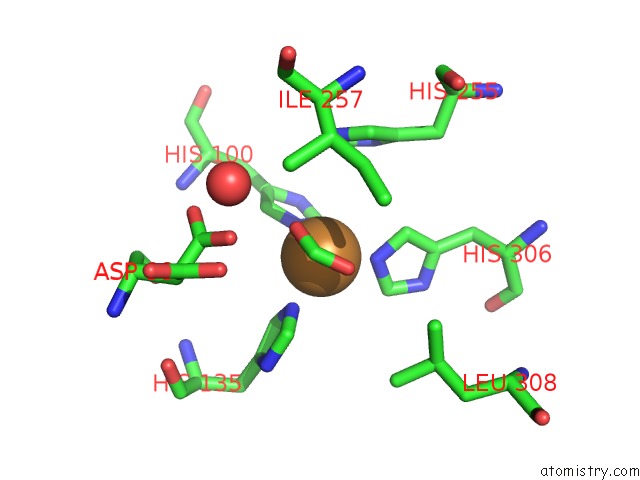
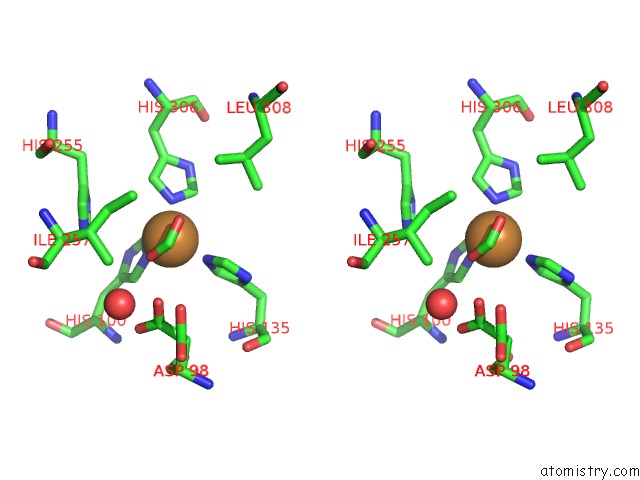
Copper binding site 2 out of 6 in 2pp8
Go back to
Copper binding site 2 out
of 6 in the Formate Bound to Oxidized Wild Type Afnir
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Formate Bound to Oxidized Wild Type Afnir within 5.0Å range:
|
Copper binding site 3 out of 6 in 2pp8
Go back to
Copper binding site 3 out
of 6 in the Formate Bound to Oxidized Wild Type Afnir
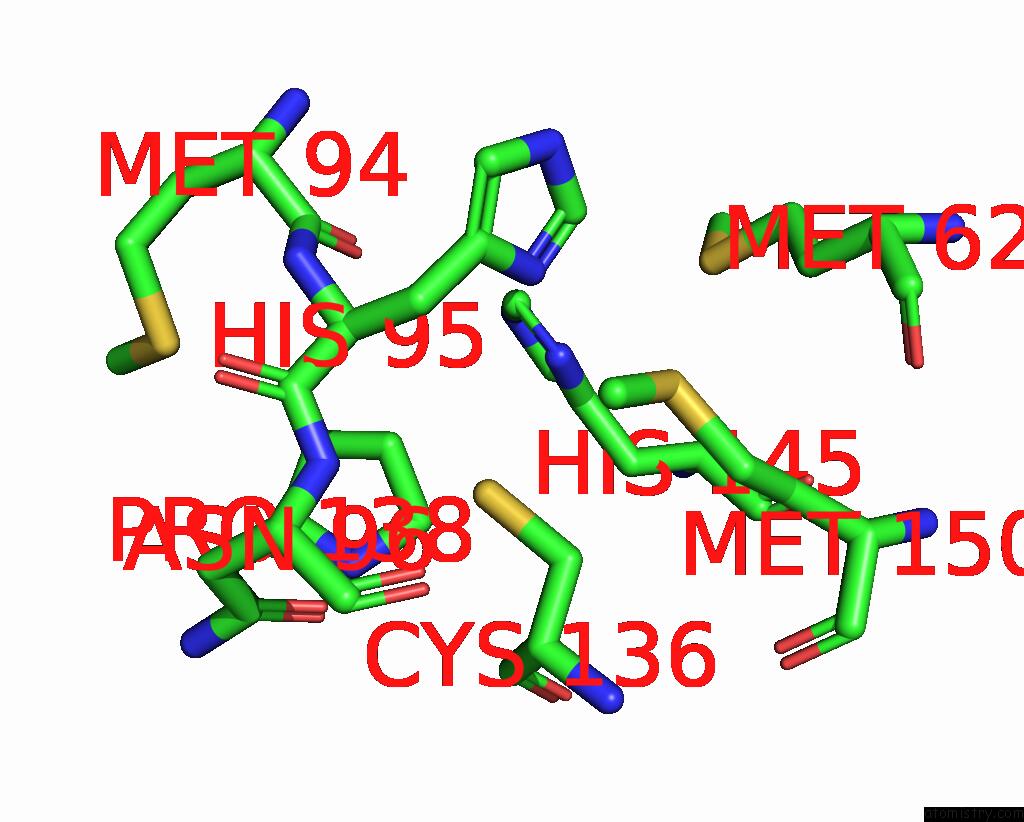
Mono view
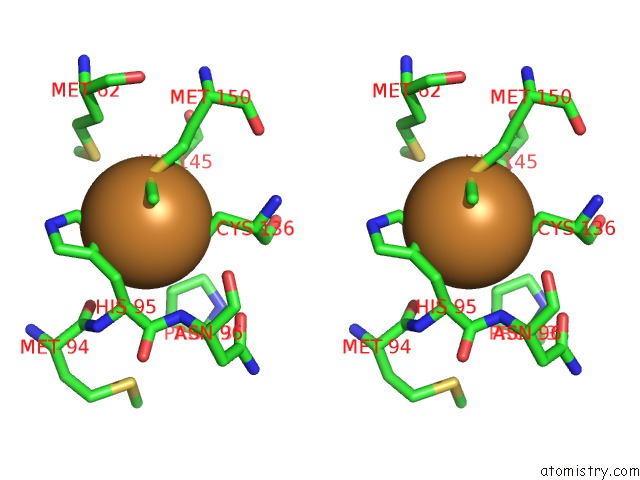
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Formate Bound to Oxidized Wild Type Afnir within 5.0Å range:
|
Copper binding site 4 out of 6 in 2pp8
Go back to
Copper binding site 4 out
of 6 in the Formate Bound to Oxidized Wild Type Afnir
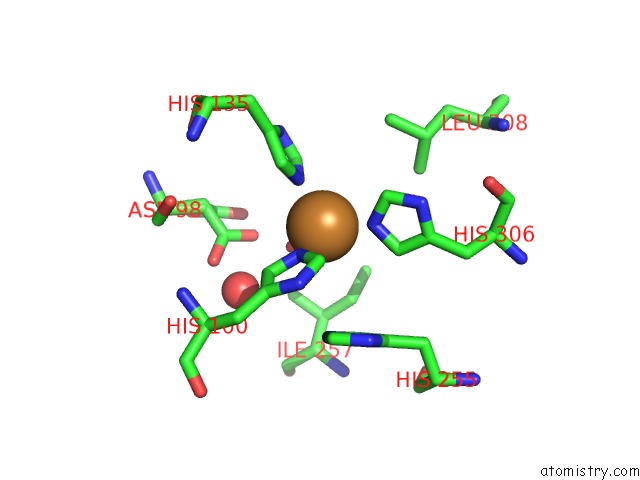
Mono view
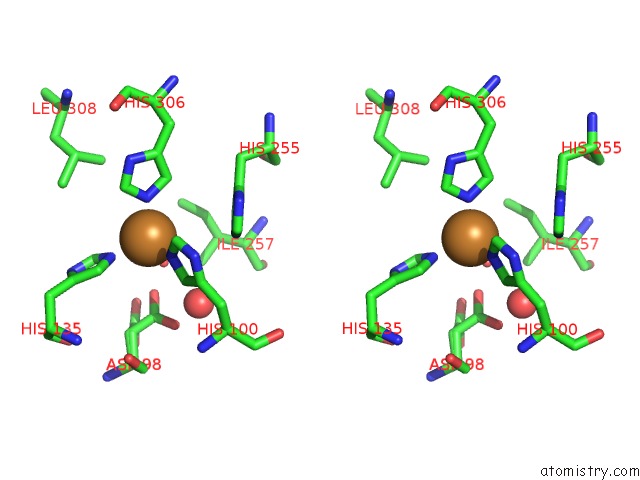
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Formate Bound to Oxidized Wild Type Afnir within 5.0Å range:
|
Copper binding site 5 out of 6 in 2pp8
Go back to
Copper binding site 5 out
of 6 in the Formate Bound to Oxidized Wild Type Afnir
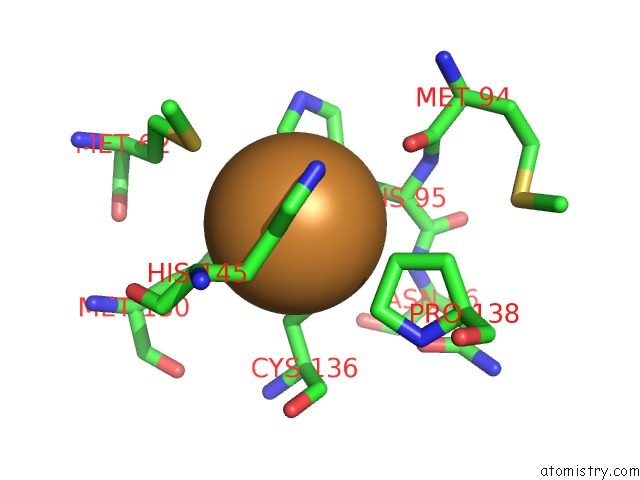
Mono view
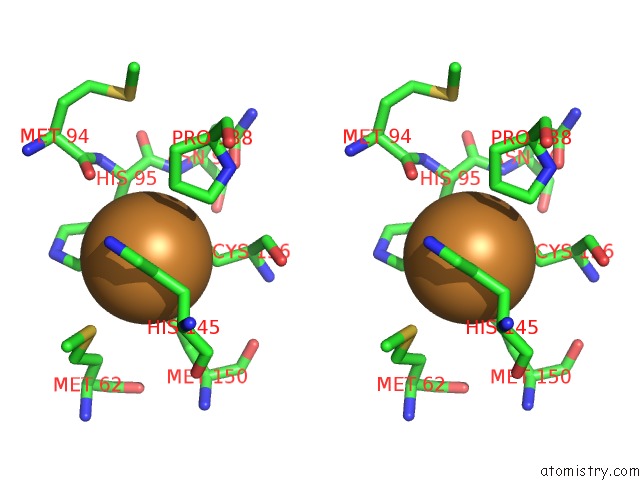
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of Formate Bound to Oxidized Wild Type Afnir within 5.0Å range:
|
Copper binding site 6 out of 6 in 2pp8
Go back to
Copper binding site 6 out
of 6 in the Formate Bound to Oxidized Wild Type Afnir
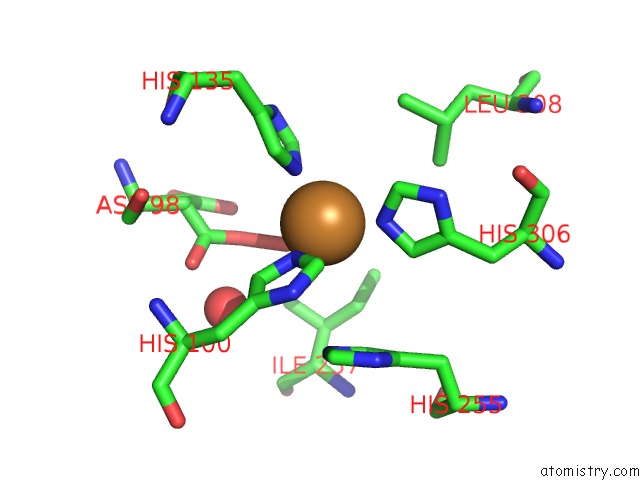
Mono view
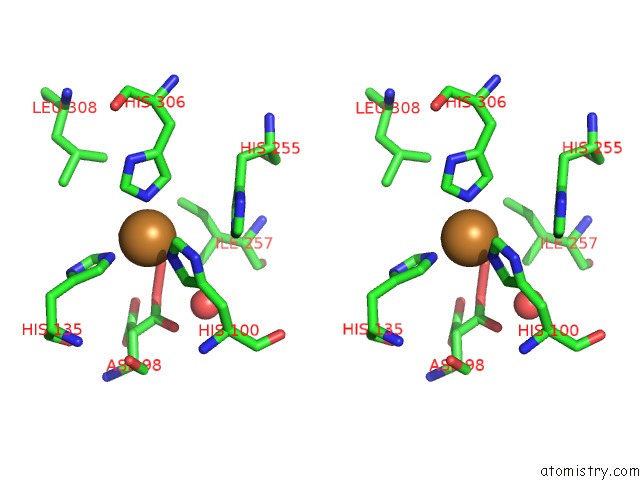
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of Formate Bound to Oxidized Wild Type Afnir within 5.0Å range:
|
Reference:
E.I.Tocheva,
L.D.Eltis,
M.E.Murphy.
Conserved Active Site Residues Limit Inhibition of A Copper-Containing Nitrite Reductase By Small Molecules. Biochemistry V. 47 4452 2008.
ISSN: ISSN 0006-2960
PubMed: 18358002
DOI: 10.1021/BI7020537
Page generated: Tue Jul 30 23:55:48 2024
ISSN: ISSN 0006-2960
PubMed: 18358002
DOI: 10.1021/BI7020537
Last articles
Cl in 4Z7OCl in 4Z6Z
Cl in 4Z6W
Cl in 4Z6V
Cl in 4Z7N
Cl in 4Z79
Cl in 4Z6U
Cl in 4Z6T
Cl in 4Z6R
Cl in 4Z6S