Copper »
PDB 2cj3-2foy »
2eib »
Copper in PDB 2eib: Crystal Structure of Galactose Oxidase, W290H Mutant
Enzymatic activity of Crystal Structure of Galactose Oxidase, W290H Mutant
All present enzymatic activity of Crystal Structure of Galactose Oxidase, W290H Mutant:
1.1.3.9;
1.1.3.9;
Protein crystallography data
The structure of Crystal Structure of Galactose Oxidase, W290H Mutant, PDB code: 2eib
was solved by
S.E.Phillips,
M.J.Mcpherson,
P.F.Knowles,
C.Wilmot,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 2.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 98.000, 89.400, 86.700, 90.00, 117.80, 90.00 |
| R / Rfree (%) | n/a / n/a |
Other elements in 2eib:
The structure of Crystal Structure of Galactose Oxidase, W290H Mutant also contains other interesting chemical elements:
| Sodium | (Na) | 1 atom |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of Galactose Oxidase, W290H Mutant
(pdb code 2eib). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total only one binding site of Copper was determined in the Crystal Structure of Galactose Oxidase, W290H Mutant, PDB code: 2eib:
In total only one binding site of Copper was determined in the Crystal Structure of Galactose Oxidase, W290H Mutant, PDB code: 2eib:
Copper binding site 1 out of 1 in 2eib
Go back to
Copper binding site 1 out
of 1 in the Crystal Structure of Galactose Oxidase, W290H Mutant
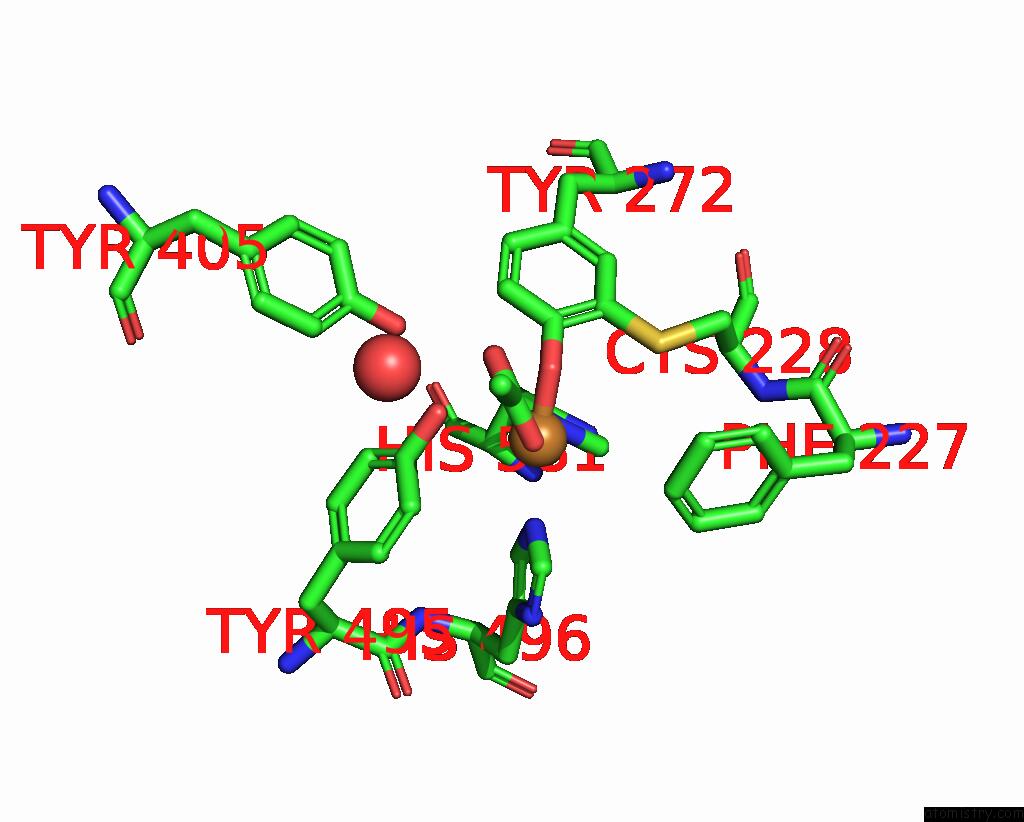
Mono view
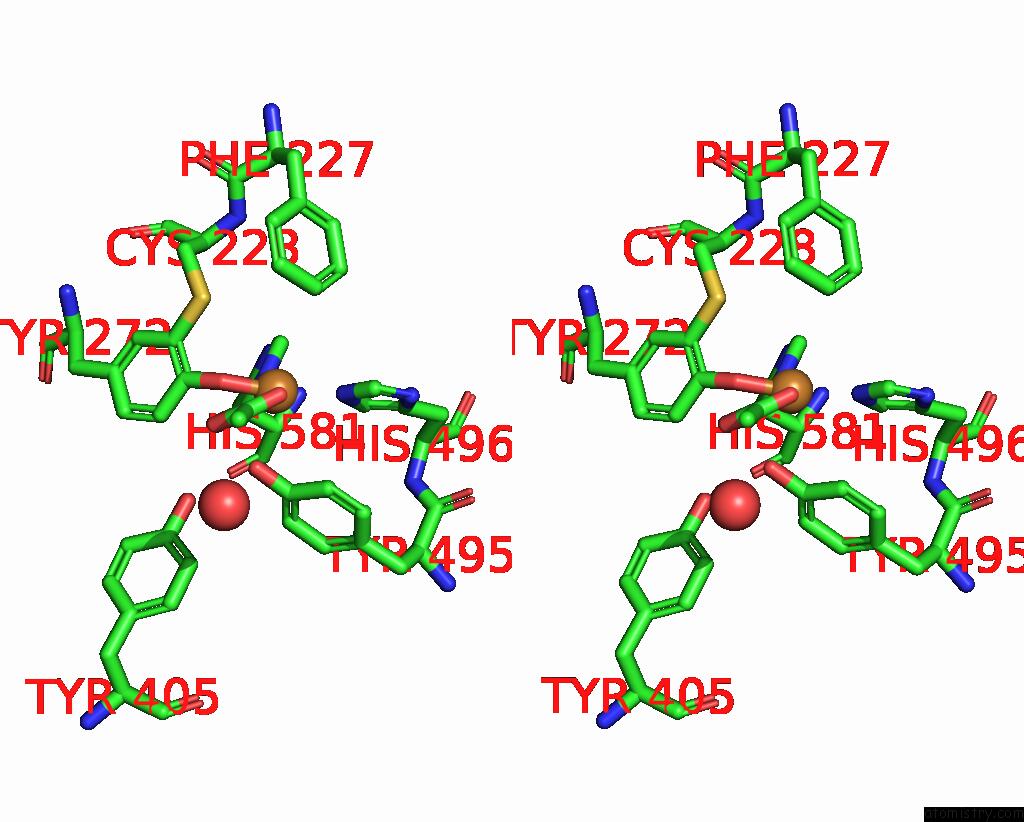
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of Galactose Oxidase, W290H Mutant within 5.0Å range:
|
Reference:
M.S.Rogers,
E.M.Tyler,
N.Akyumani,
C.R.Kurtis,
R.K.Spooner,
S.E.Deacon,
S.Tamber,
S.J.Firbank,
K.Mahmoud,
P.F.Knowles,
S.E.Phillips,
M.J.Mcpherson,
D.M.Dooley.
The Stacking Tryptophan of Galactose Oxidase: A Second-Coordination Sphere Residue That Has Profound Effects on Tyrosyl Radical Behavior and Enzyme Catalysis Biochemistry V. 46 4606 2007.
ISSN: ISSN 0006-2960
PubMed: 17385891
DOI: 10.1021/BI062139D
Page generated: Mon Jul 14 00:56:41 2025
ISSN: ISSN 0006-2960
PubMed: 17385891
DOI: 10.1021/BI062139D
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO