Copper »
PDB 2cj3-2foy »
2cwt »
Copper in PDB 2cwt: Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis
Enzymatic activity of Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis
All present enzymatic activity of Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis:
1.4.3.6;
1.4.3.6;
Protein crystallography data
The structure of Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis, PDB code: 2cwt
was solved by
Y.C.Chiu,
T.Okajima,
T.Murakawa,
M.Uchida,
M.Taki,
S.Hirota,
M.Kim,
H.Yamaguchi,
Y.Kawano,
N.Kamiya,
S.Kuroda,
H.Hayashi,
Y.Yamamoto,
K.Tanizawa,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.02 / 1.82 |
| Space group | I 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 157.668, 63.840, 184.453, 90.00, 112.40, 90.00 |
| R / Rfree (%) | 19.1 / 20.4 |
Copper Binding Sites:
The binding sites of Copper atom in the Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis
(pdb code 2cwt). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis, PDB code: 2cwt:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis, PDB code: 2cwt:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 2cwt
Go back to
Copper binding site 1 out
of 2 in the Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis
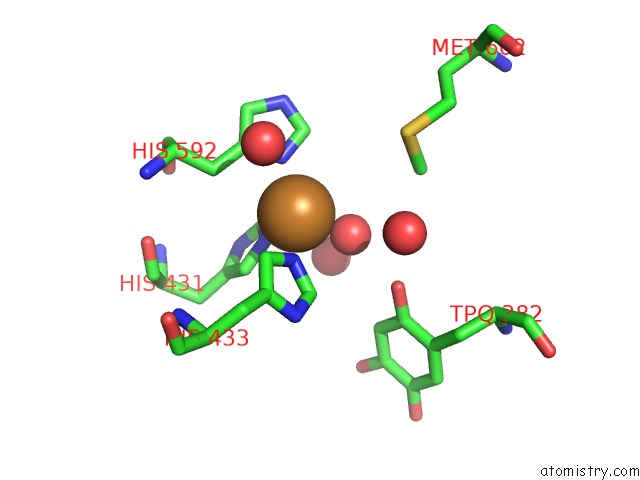
Mono view
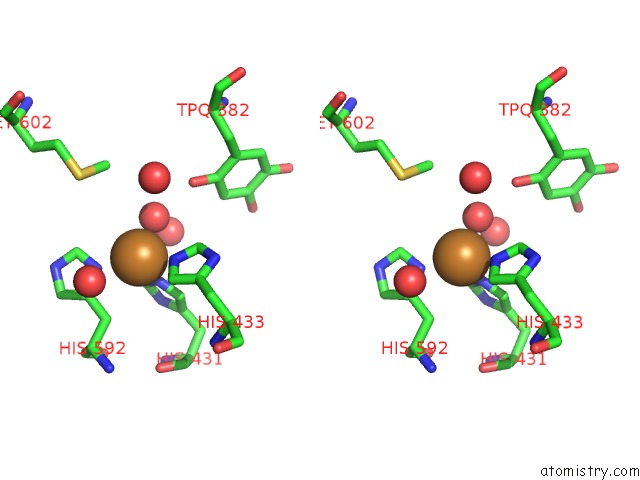
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis within 5.0Å range:
|
Copper binding site 2 out of 2 in 2cwt
Go back to
Copper binding site 2 out
of 2 in the Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis
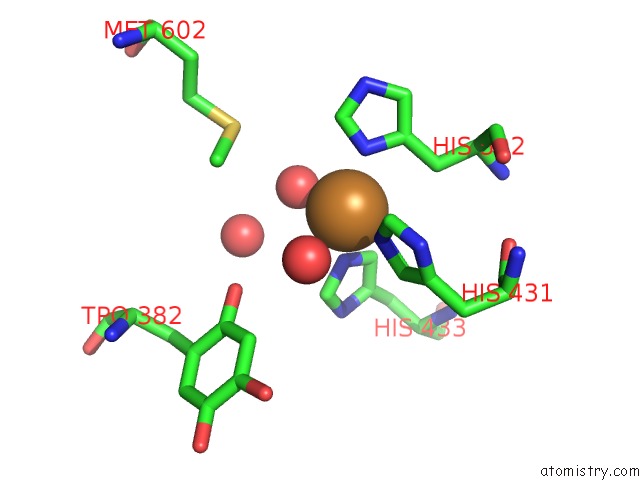
Mono view
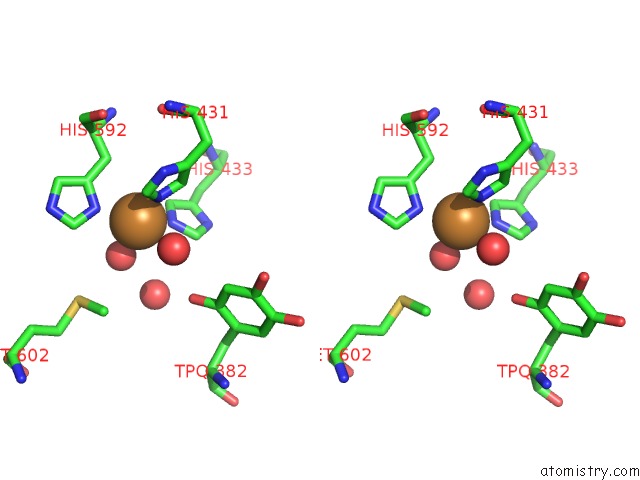
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Catalytic Base Deletion in Copper Amine Oxidase From Arthrobacter Globiformis within 5.0Å range:
|
Reference:
Y.C.Chiu,
T.Okajima,
T.Murakawa,
M.Uchida,
M.Taki,
S.Hirota,
M.Kim,
H.Yamaguchi,
Y.Kawano,
N.Kamiya,
S.Kuroda,
H.Hayashi,
Y.Yamamoto,
K.Tanizawa.
Kinetic and Structural Studies on the Catalytic Role of the Aspartic Acid Residue Conserved in Copper Amine Oxidase(,) Biochemistry V. 45 4105 2006.
ISSN: ISSN 0006-2960
PubMed: 16566584
DOI: 10.1021/BI052464L
Page generated: Mon Jul 14 00:53:30 2025
ISSN: ISSN 0006-2960
PubMed: 16566584
DOI: 10.1021/BI052464L
Last articles
F in 4CKRF in 4CFN
F in 4CLJ
F in 4CLI
F in 4CK7
F in 4CK6
F in 4CKA
F in 4CGN
F in 4C7O
F in 4CFT