Copper »
PDB 1tmx-1x9l »
1w77 »
Copper in PDB 1w77: 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
Enzymatic activity of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
All present enzymatic activity of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana:
2.7.7.60;
2.7.7.60;
Protein crystallography data
The structure of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana, PDB code: 1w77
was solved by
M.Gabrielsen,
J.Kaiser,
F.Rohdich,
W.Eisenreich,
A.Bacher,
C.S.Bond,
W.N.Hunter,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 27.95 / 2.00 |
| Space group | H 3 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 74.496, 74.496, 223.026, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 23.2 / 34.9 |
Other elements in 1w77:
The structure of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana also contains other interesting chemical elements:
| Cadmium | (Cd) | 1 atom |
Copper Binding Sites:
The binding sites of Copper atom in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
(pdb code 1w77). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 4 binding sites of Copper where determined in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana, PDB code: 1w77:
Jump to Copper binding site number: 1; 2; 3; 4;
In total 4 binding sites of Copper where determined in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana, PDB code: 1w77:
Jump to Copper binding site number: 1; 2; 3; 4;
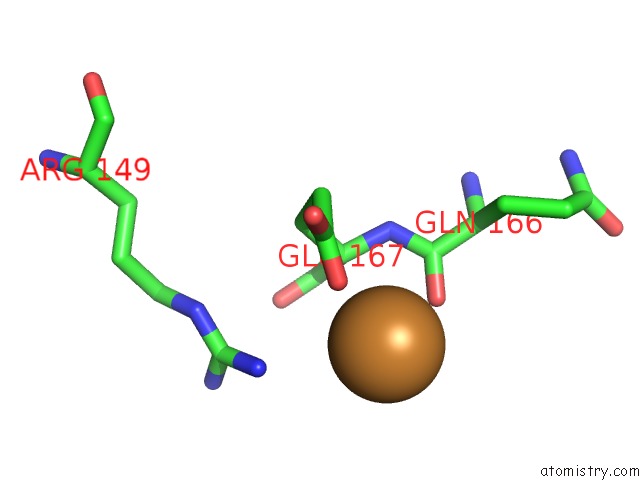
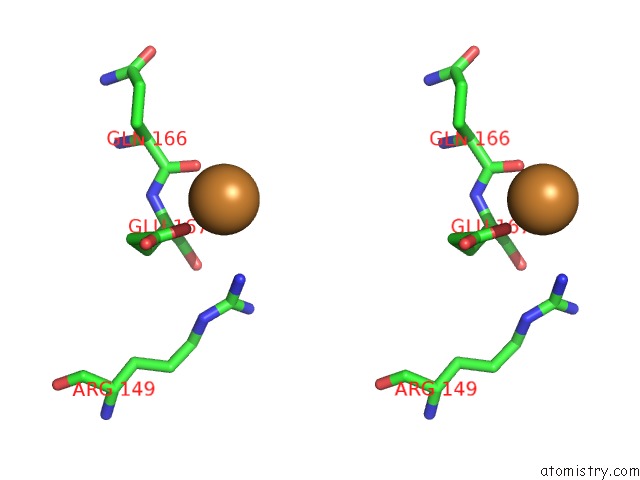
Copper binding site 1 out of 4 in 1w77
Go back to
Copper binding site 1 out
of 4 in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana within 5.0Å range:
|
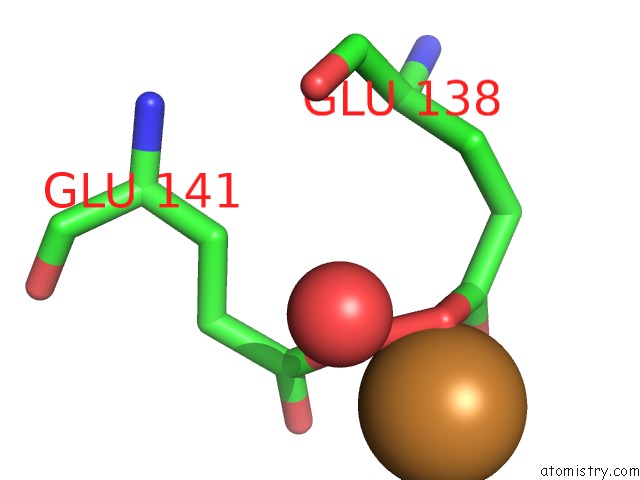
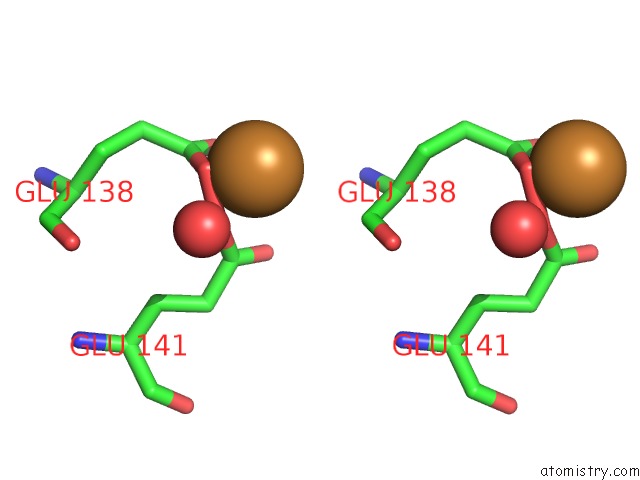
Copper binding site 2 out of 4 in 1w77
Go back to
Copper binding site 2 out
of 4 in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana within 5.0Å range:
|
Copper binding site 3 out of 4 in 1w77
Go back to
Copper binding site 3 out
of 4 in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
Mono view
Stereo pair view
Mono view
Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana within 5.0Å range:
|
Copper binding site 4 out of 4 in 1w77
Go back to
Copper binding site 4 out
of 4 in the 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana
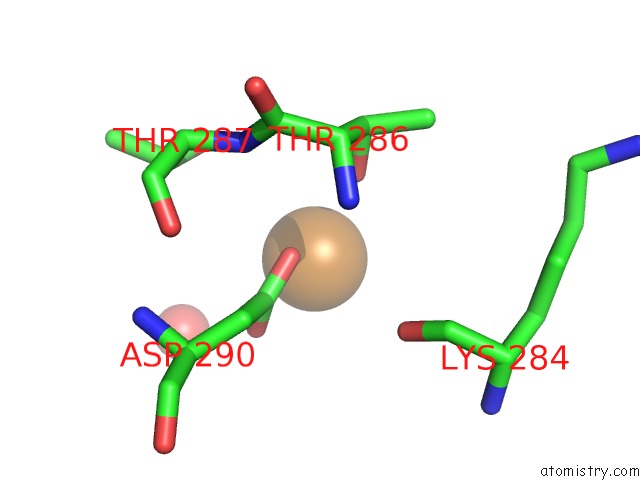
Mono view
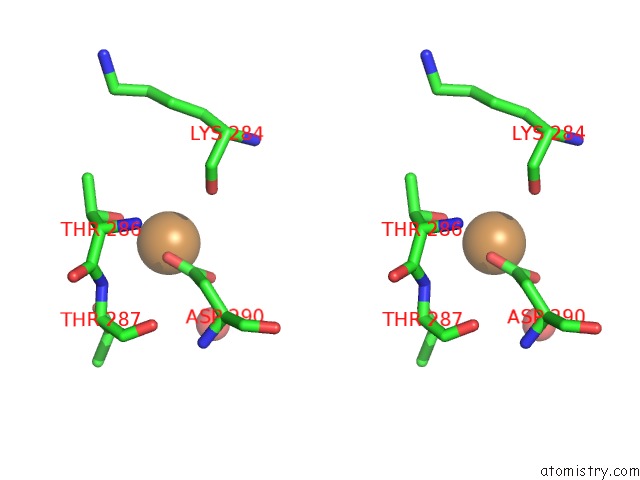
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of 2C-Methyl-D-Erythritol 4-Phosphate Cytidylyltransferase (Ispd) From Arabidopsis Thaliana within 5.0Å range:
|
Reference:
M.Gabrielsen,
J.Kaiser,
F.Rohdich,
W.Eisenreich,
R.Laupitz,
A.Bacher,
C.S.Bond,
W.N.Hunter.
The Crystal Structure of A Plant 2C-Methyl-D- Erythritol 4-Phosphate Cytidylyltransferase Exhibits A Distinct Quaternary Structure Compared to Bacterial Homologues and A Possible Role in Feedback Regulation For Cytidine Monophosphate. Febs J. V. 273 1065 2006.
ISSN: ISSN 1742-464X
PubMed: 16478479
DOI: 10.1111/J.1742-4658.2006.05133.X
Page generated: Mon Jul 14 00:34:26 2025
ISSN: ISSN 1742-464X
PubMed: 16478479
DOI: 10.1111/J.1742-4658.2006.05133.X
Last articles
F in 7M8OF in 7M8P
F in 7M7D
F in 7M63
F in 7M7N
F in 7M5Y
F in 7M5X
F in 7M5Z
F in 7M2N
F in 7M4V