Copper »
PDB 1tmx-1x9l »
1w4n »
Copper in PDB 1w4n: Agao Covalent Complex with Tranylcypromine
Enzymatic activity of Agao Covalent Complex with Tranylcypromine
All present enzymatic activity of Agao Covalent Complex with Tranylcypromine:
1.4.3.6;
1.4.3.6;
Protein crystallography data
The structure of Agao Covalent Complex with Tranylcypromine, PDB code: 1w4n
was solved by
A.P.Duff,
D.M.Trambaiolo,
D.B.Langley,
G.A.Juda,
E.M.Shepard,
D.M.Dooley,
H.C.Freeman,
J.M.Guss,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.28 / 1.65 |
| Space group | I 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 158.113, 63.001, 184.490, 90.00, 112.04, 90.00 |
| R / Rfree (%) | 17.1 / 19.9 |
Other elements in 1w4n:
The structure of Agao Covalent Complex with Tranylcypromine also contains other interesting chemical elements:
| Sodium | (Na) | 2 atoms |
Copper Binding Sites:
The binding sites of Copper atom in the Agao Covalent Complex with Tranylcypromine
(pdb code 1w4n). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Agao Covalent Complex with Tranylcypromine, PDB code: 1w4n:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Agao Covalent Complex with Tranylcypromine, PDB code: 1w4n:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 1w4n
Go back to
Copper binding site 1 out
of 2 in the Agao Covalent Complex with Tranylcypromine
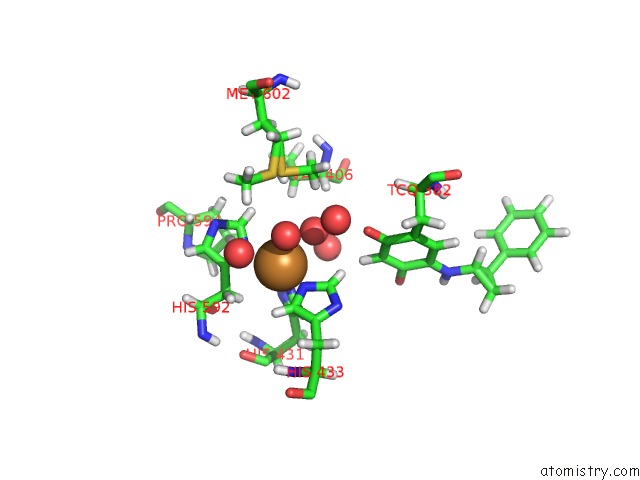
Mono view
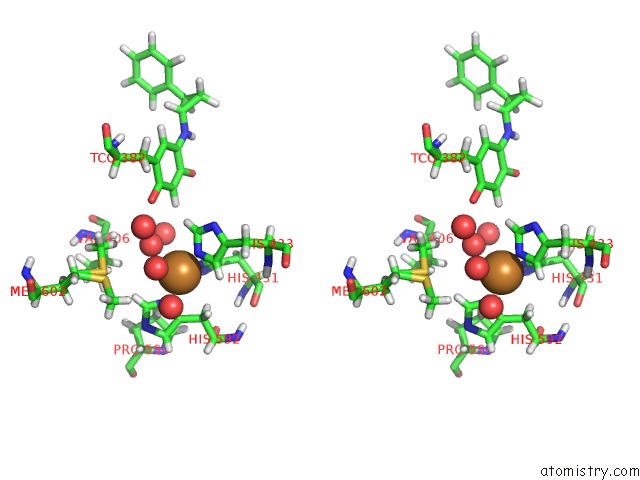
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Agao Covalent Complex with Tranylcypromine within 5.0Å range:
|
Copper binding site 2 out of 2 in 1w4n
Go back to
Copper binding site 2 out
of 2 in the Agao Covalent Complex with Tranylcypromine
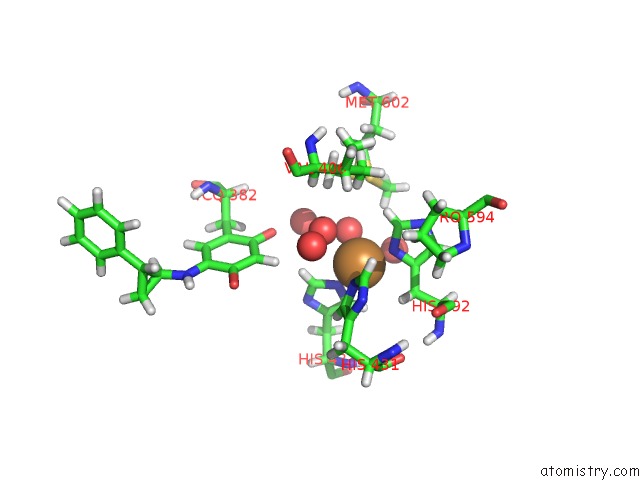
Mono view
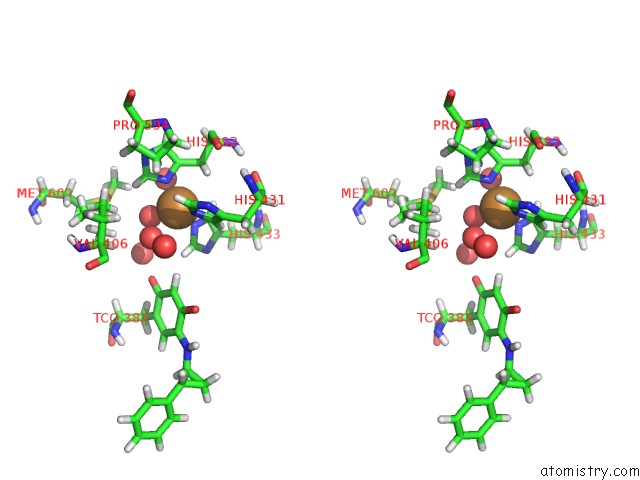
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Agao Covalent Complex with Tranylcypromine within 5.0Å range:
|
Reference:
D.B.Langley,
D.M.Trambaiolo,
A.P.Duff,
D.M.Dooley,
H.C.Freeman,
J.M.Guss.
Complexes of the Copper-Containing Amine Oxidase From Arthrobacter Globiformis with the Inhibitors Benzylhydrazine and Tranylcypromine. Acta Crystallogr.,Sect.F V. 64 577 2008.
ISSN: ESSN 1744-3091
PubMed: 18607080
DOI: 10.1107/S174430910801556X
Page generated: Tue Jul 30 22:54:16 2024
ISSN: ESSN 1744-3091
PubMed: 18607080
DOI: 10.1107/S174430910801556X
Last articles
Cl in 7WFRCl in 7WF8
Cl in 7WEL
Cl in 7WE4
Cl in 7WCX
Cl in 7WCW
Cl in 7WCT
Cl in 7WCV
Cl in 7WAO
Cl in 7WBF