Copper »
PDB 1oe2-1rjp »
1pzs »
Copper in PDB 1pzs: Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution
Enzymatic activity of Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution
All present enzymatic activity of Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution:
1.15.1.1;
1.15.1.1;
Protein crystallography data
The structure of Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution, PDB code: 1pzs
was solved by
K.Djinovic-Carugo,
L.Spagnolo,
I.Toro,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 27.95 / 1.63 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 70.040, 58.420, 51.390, 90.00, 126.99, 90.00 |
| R / Rfree (%) | 15 / 19 |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution
(pdb code 1pzs). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total only one binding site of Copper was determined in the Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution, PDB code: 1pzs:
In total only one binding site of Copper was determined in the Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution, PDB code: 1pzs:
Copper binding site 1 out of 1 in 1pzs
Go back to
Copper binding site 1 out
of 1 in the Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution
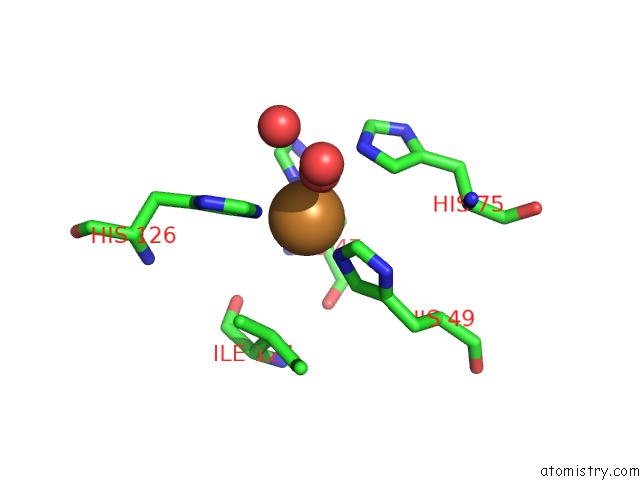
Mono view
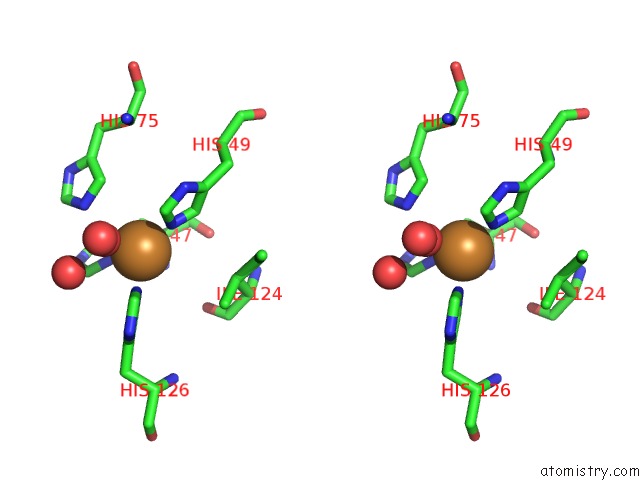
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of A Cu-Zn Superoxide Dismutase From Mycobacterium Tuberculosis at 1.63 Resolution within 5.0Å range:
|
Reference:
L.Spagnolo,
I.Toro,
M.D'orazio,
P.O'neill,
J.Z.Pedersen,
O.Carugo,
G.Rotilio,
A.Battistoni,
K.Djinovic-Carugo.
Unique Features of the Sodc-Encoded Superoxide Dismutase From Mycobacterium Tuberculosis, A Fully Functional Copper-Containing Enzyme Lacking Zinc in the Active Site. J.Biol.Chem. V. 279 33447 2004.
ISSN: ISSN 0021-9258
PubMed: 15155722
DOI: 10.1074/JBC.M404699200
Page generated: Tue Jul 30 22:36:36 2024
ISSN: ISSN 0021-9258
PubMed: 15155722
DOI: 10.1074/JBC.M404699200
Last articles
Cl in 5C02Cl in 5BXN
Cl in 5BXY
Cl in 5BYF
Cl in 5BYE
Cl in 5BXL
Cl in 5BXU
Cl in 5BWV
Cl in 5BWX
Cl in 5BXA