Copper »
PDB 1oe2-1rjp »
1pf3 »
Copper in PDB 1pf3: Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo
Protein crystallography data
The structure of Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo, PDB code: 1pf3
was solved by
S.A.Roberts,
G.F.Wildner,
G.Grass,
A.Weichsel,
A.Ambrus,
C.Rensing,
W.R.Montfort,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.50 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 50.445, 91.193, 53.905, 90.00, 102.35, 90.00 |
| R / Rfree (%) | 19.1 / 22.8 |
Other elements in 1pf3:
The structure of Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo also contains other interesting chemical elements:
| Chlorine | (Cl) | 1 atom |
Copper Binding Sites:
The binding sites of Copper atom in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo
(pdb code 1pf3). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 4 binding sites of Copper where determined in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo, PDB code: 1pf3:
Jump to Copper binding site number: 1; 2; 3; 4;
In total 4 binding sites of Copper where determined in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo, PDB code: 1pf3:
Jump to Copper binding site number: 1; 2; 3; 4;
Copper binding site 1 out of 4 in 1pf3
Go back to
Copper binding site 1 out
of 4 in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo
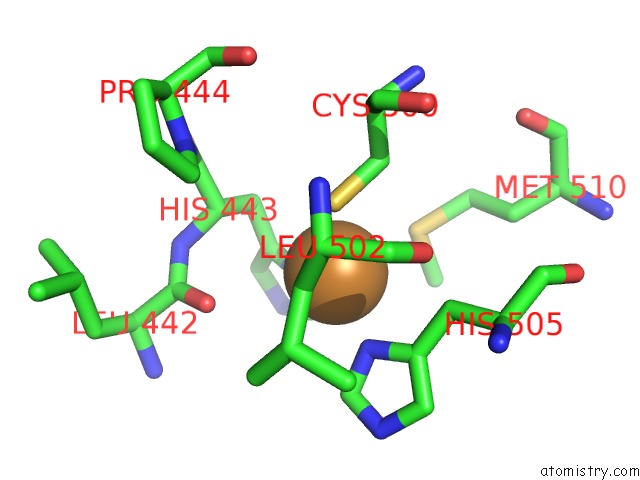
Mono view
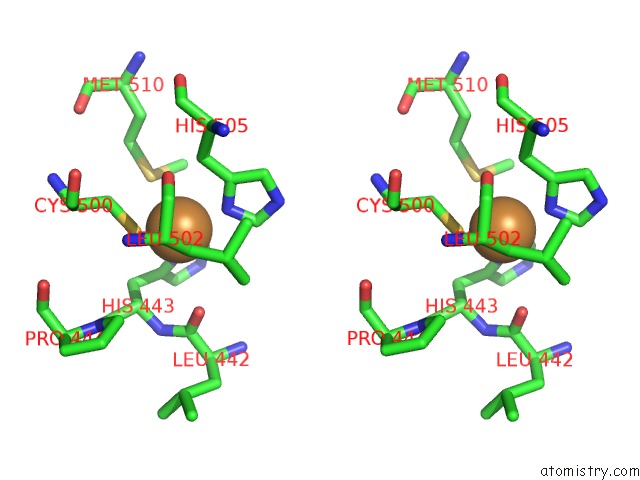
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo within 5.0Å range:
|
Copper binding site 2 out of 4 in 1pf3
Go back to
Copper binding site 2 out
of 4 in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo
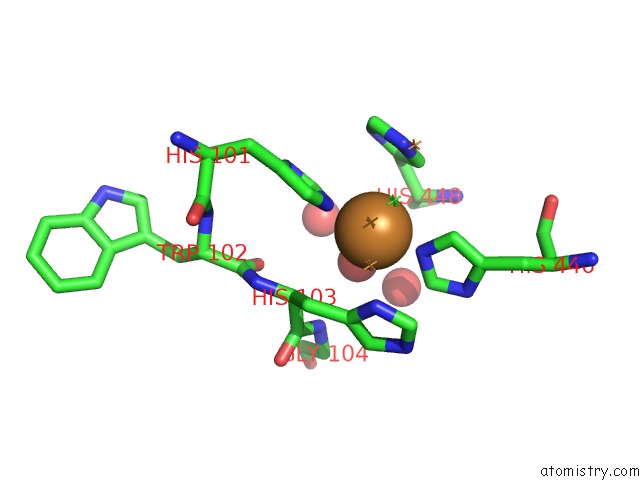
Mono view
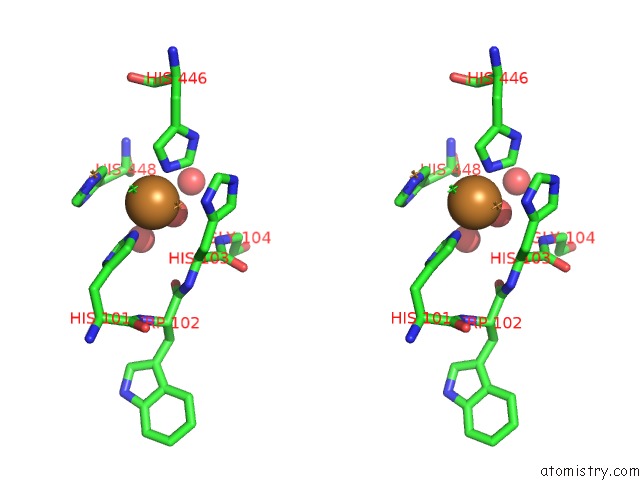
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo within 5.0Å range:
|
Copper binding site 3 out of 4 in 1pf3
Go back to
Copper binding site 3 out
of 4 in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo
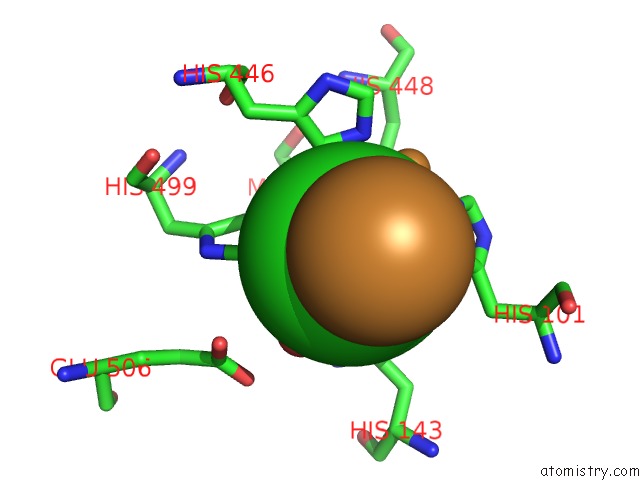
Mono view
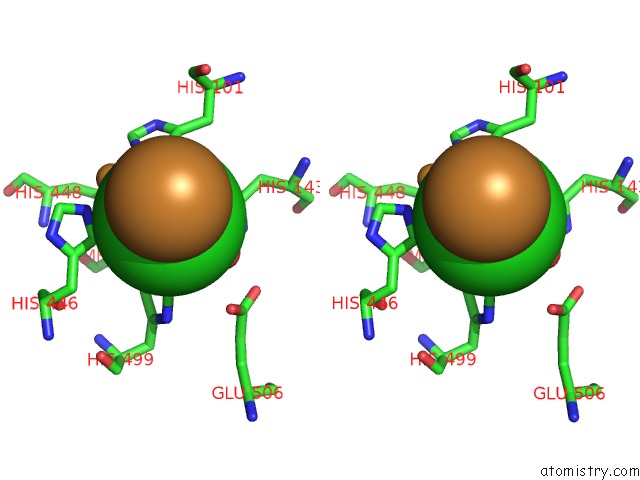
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo within 5.0Å range:
|
Copper binding site 4 out of 4 in 1pf3
Go back to
Copper binding site 4 out
of 4 in the Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo
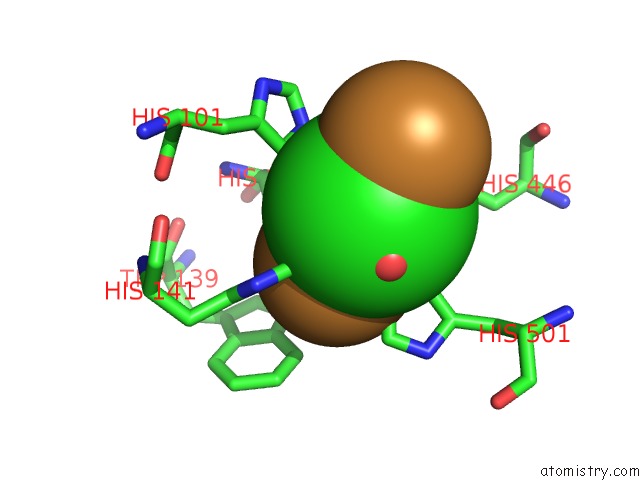
Mono view
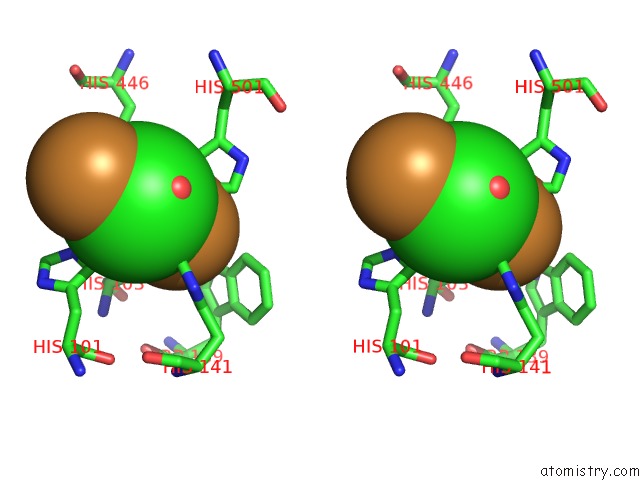
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of Crystal Structure of the M441L Mutant of the Multicopper Oxidase Cueo within 5.0Å range:
|
Reference:
S.A.Roberts,
G.F.Wildner,
G.Grass,
A.Weichsel,
A.Ambrus,
C.Rensing,
W.R.Montfort.
A Labile Regulatory Copper Ion Lies Near the T1 Copper Site in the Multicopper Oxidase Cueo. J.Biol.Chem. V. 278 31958 2003.
ISSN: ISSN 0021-9258
PubMed: 12794077
DOI: 10.1074/JBC.M302963200
Page generated: Mon Jul 14 00:16:21 2025
ISSN: ISSN 0021-9258
PubMed: 12794077
DOI: 10.1074/JBC.M302963200
Last articles
Fe in 2YXOFe in 2YRS
Fe in 2YXC
Fe in 2YNM
Fe in 2YVJ
Fe in 2YP1
Fe in 2YU2
Fe in 2YU1
Fe in 2YQB
Fe in 2YOO