Copper »
PDB 1hc1-1jvo »
1jvl »
Copper in PDB 1jvl: Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether
Protein crystallography data
The structure of Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether, PDB code: 1jvl
was solved by
I.M.C.Van Amsterdam,
M.Ubbink,
O.Einsle,
A.Messerschmidt,
A.Merli,
D.Cavazzini,
G.L.Rossi,
G.W.Canters,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 2.00 |
| Space group | P 61 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.605, 48.605, 284.809, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 19 / 22.7 |
Other elements in 1jvl:
The structure of Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether also contains other interesting chemical elements:
| Nickel | (Ni) | 2 atoms |
Copper Binding Sites:
The binding sites of Copper atom in the Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether
(pdb code 1jvl). This binding sites where shown within
5.0 Angstroms radius around Copper atom.
In total 2 binding sites of Copper where determined in the Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether, PDB code: 1jvl:
Jump to Copper binding site number: 1; 2;
In total 2 binding sites of Copper where determined in the Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether, PDB code: 1jvl:
Jump to Copper binding site number: 1; 2;
Copper binding site 1 out of 2 in 1jvl
Go back to
Copper binding site 1 out
of 2 in the Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether
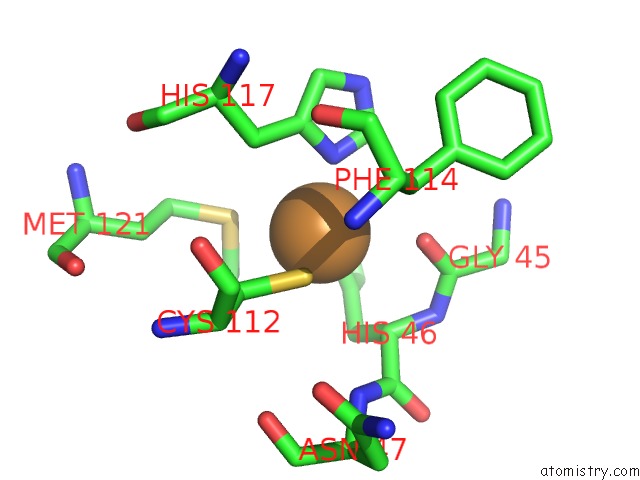
Mono view
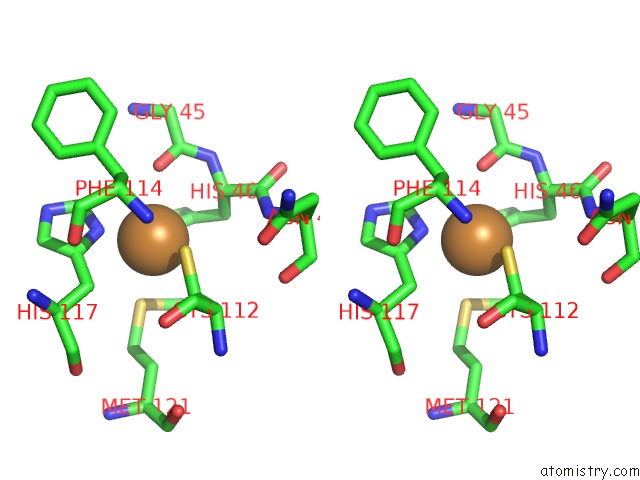
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether within 5.0Å range:
|
Copper binding site 2 out of 2 in 1jvl
Go back to
Copper binding site 2 out
of 2 in the Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether
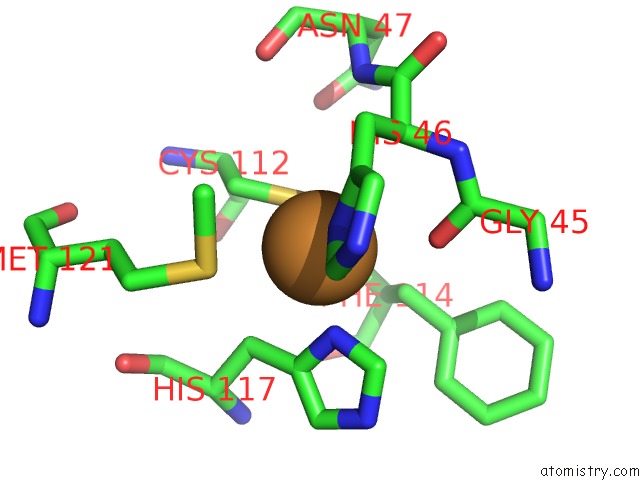
Mono view
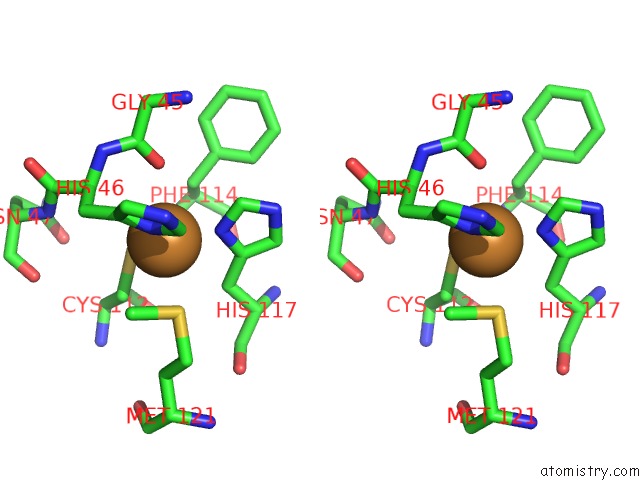
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of Azurin Dimer, Covalently Crosslinked Through Bis- Maleimidomethylether within 5.0Å range:
|
Reference:
I.M.C.Van Amsterdam,
M.Ubbink,
O.Einsle,
A.Messerschmidt,
A.Merli,
D.Cavazzini,
G.L.Rossi,
G.W.Canters.
Dramatic Modulation of Electron Transfer in Protein Complexes By Crosslinking Nat.Struct.Biol. V. 9 48 2002.
ISSN: ISSN 1072-8368
PubMed: 11740504
DOI: 10.1038/NSB736
Page generated: Tue Jul 30 22:05:41 2024
ISSN: ISSN 1072-8368
PubMed: 11740504
DOI: 10.1038/NSB736
Last articles
Cl in 5UTTCl in 5UU7
Cl in 5UU6
Cl in 5UTU
Cl in 5UU4
Cl in 5URS
Cl in 5UU3
Cl in 5URQ
Cl in 5UTN
Cl in 5USS