Copper »
PDB 1hc1-1jvo »
1hl5 »
Copper in PDB 1hl5: The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
Enzymatic activity of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
All present enzymatic activity of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase:
1.15.1.1;
1.15.1.1;
Protein crystallography data
The structure of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase, PDB code: 1hl5
was solved by
R.W.Strange,
S.Antonyuk,
M.A.Hough,
P.Doucette,
J.Rodriguez,
P.J.Hart,
L.J.Hayward,
J.S.Valentine,
S.S.Hasnain,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.8 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 76.871, 172.380, 112.450, 90.00, 93.45, 90.00 |
| R / Rfree (%) | 18.5 / 22.2 |
Other elements in 1hl5:
The structure of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase also contains other interesting chemical elements:
| Calcium | (Ca) | 3 atoms |
| Zinc | (Zn) | 18 atoms |
Copper Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 18;Binding sites:
The binding sites of Copper atom in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase (pdb code 1hl5). This binding sites where shown within 5.0 Angstroms radius around Copper atom.In total 18 binding sites of Copper where determined in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase, PDB code: 1hl5:
Jump to Copper binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Copper binding site 1 out of 18 in 1hl5
Go back to
Copper binding site 1 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
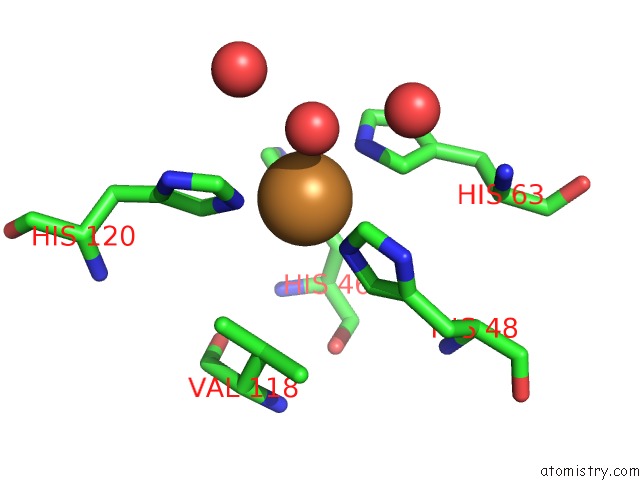
Mono view
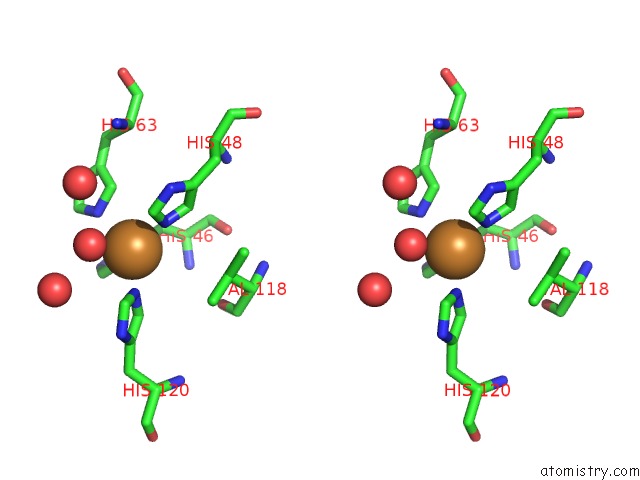
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 1 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
Copper binding site 2 out of 18 in 1hl5
Go back to
Copper binding site 2 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
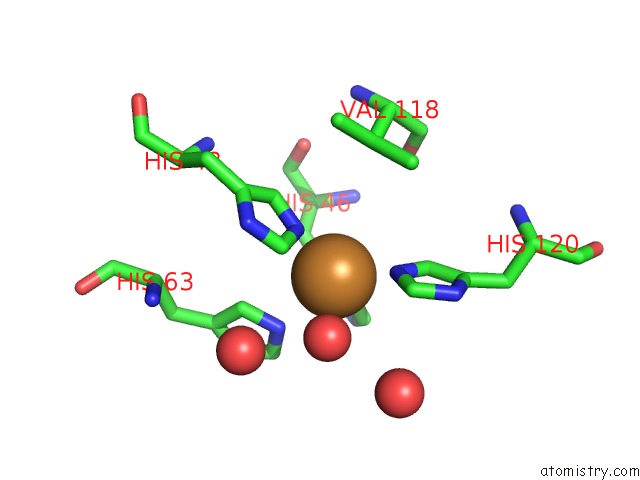
Mono view
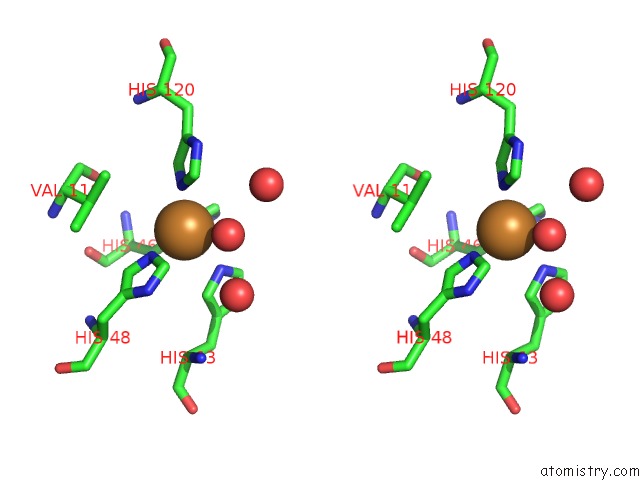
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 2 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
Copper binding site 3 out of 18 in 1hl5
Go back to
Copper binding site 3 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
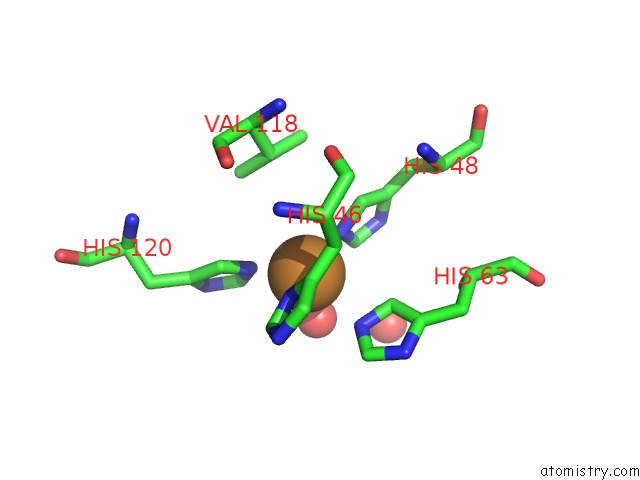
Mono view
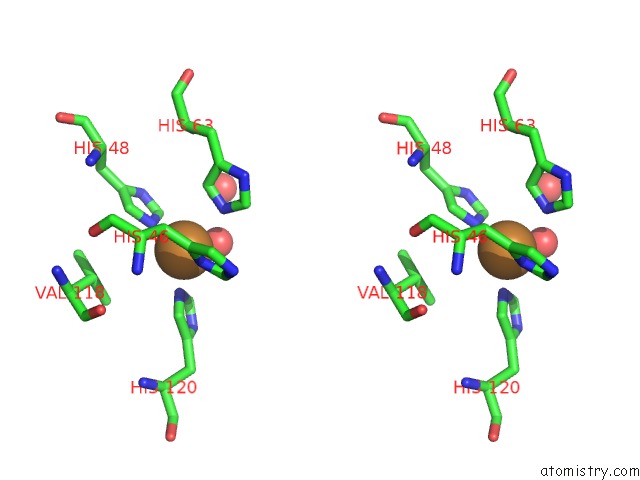
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 3 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
Copper binding site 4 out of 18 in 1hl5
Go back to
Copper binding site 4 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
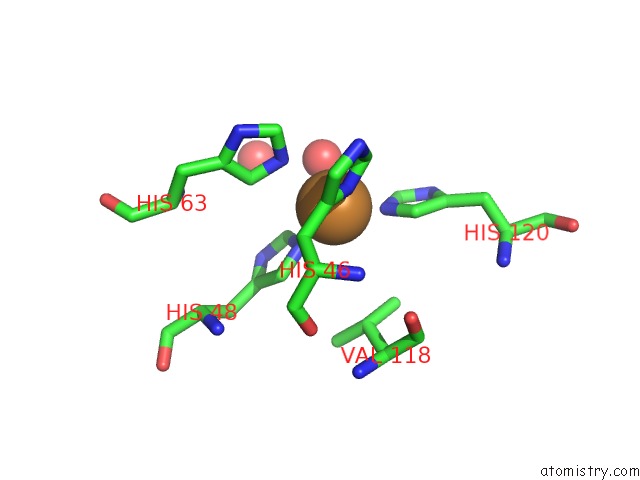
Mono view
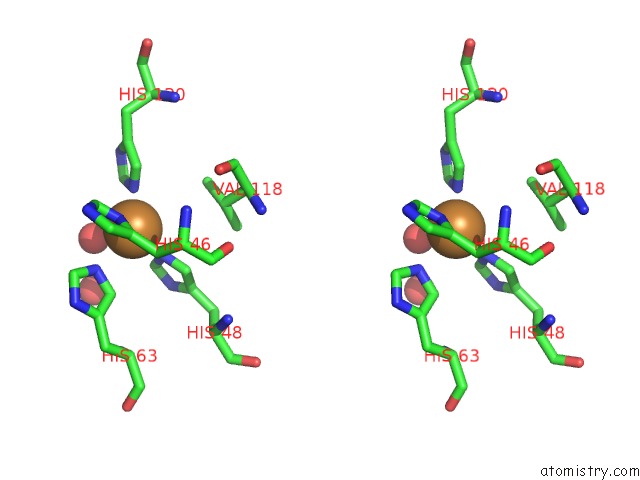
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 4 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
Copper binding site 5 out of 18 in 1hl5
Go back to
Copper binding site 5 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
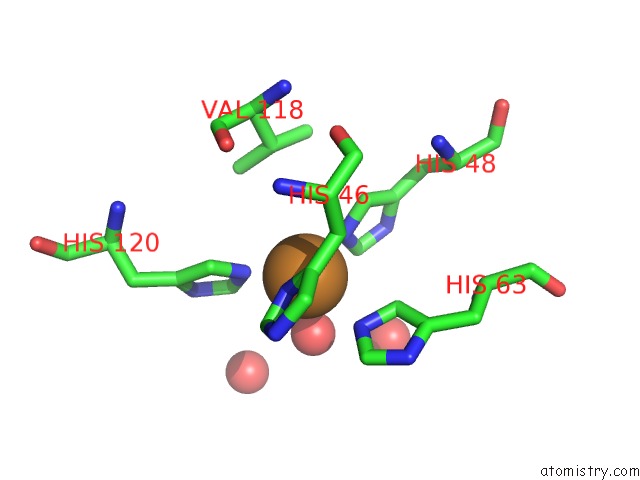
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 5 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
Copper binding site 6 out of 18 in 1hl5
Go back to
Copper binding site 6 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase

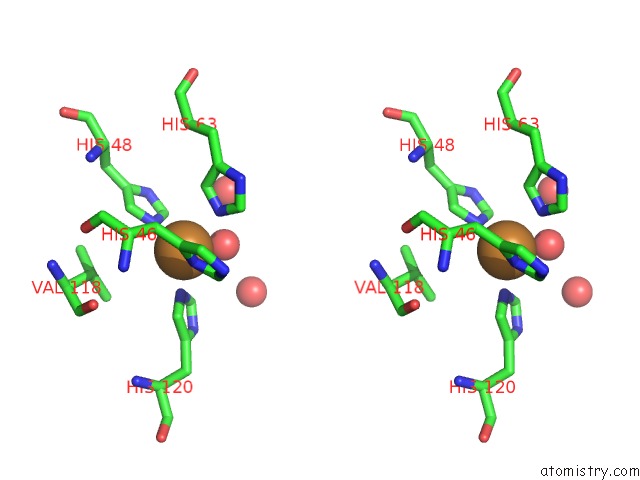
Mono view
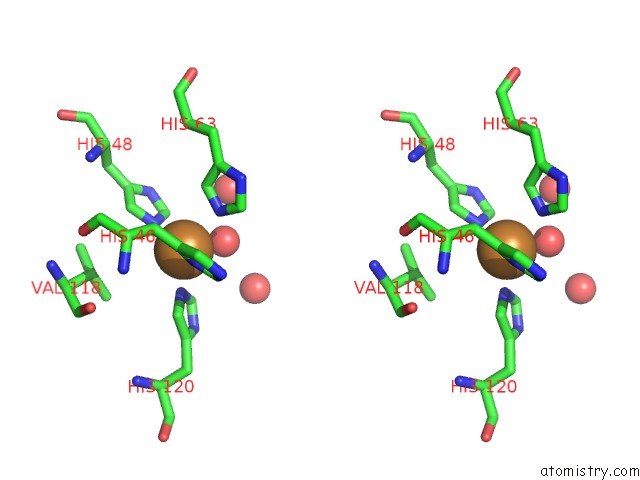
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 6 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
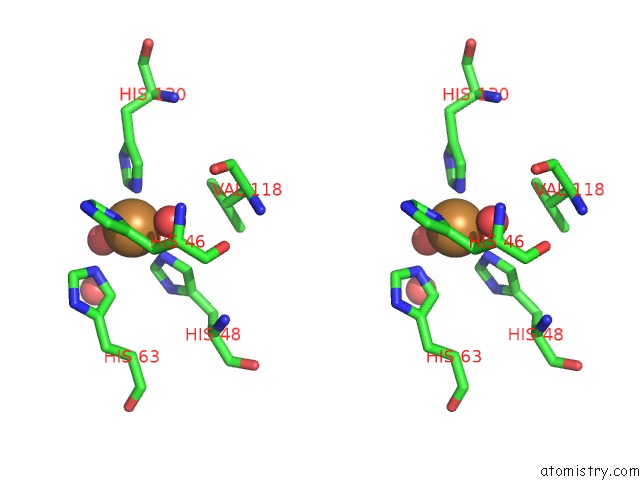
Copper binding site 7 out of 18 in 1hl5
Go back to
Copper binding site 7 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
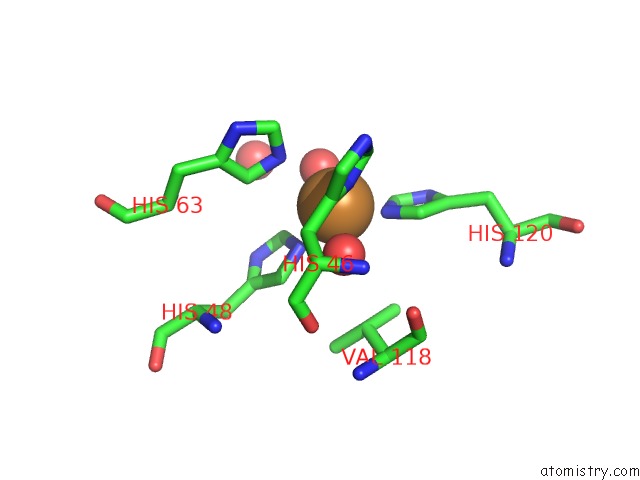
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 7 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
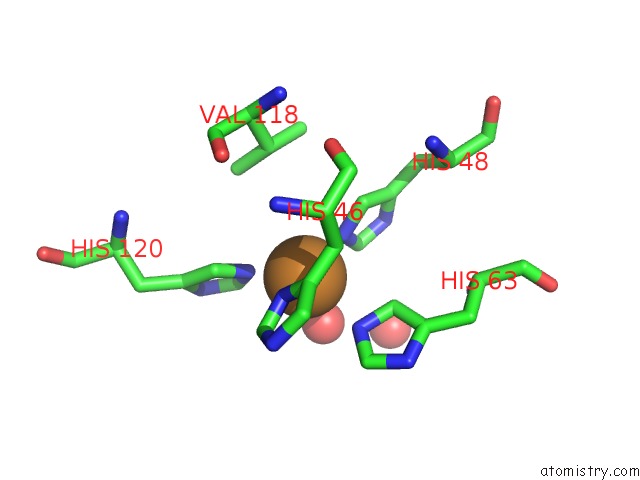
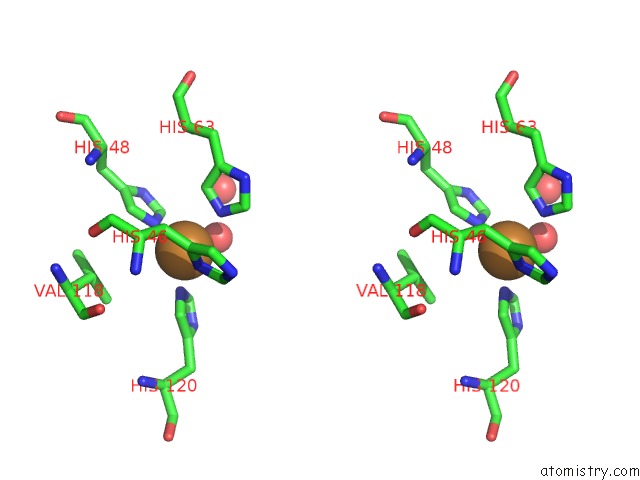
Copper binding site 8 out of 18 in 1hl5
Go back to
Copper binding site 8 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 8 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
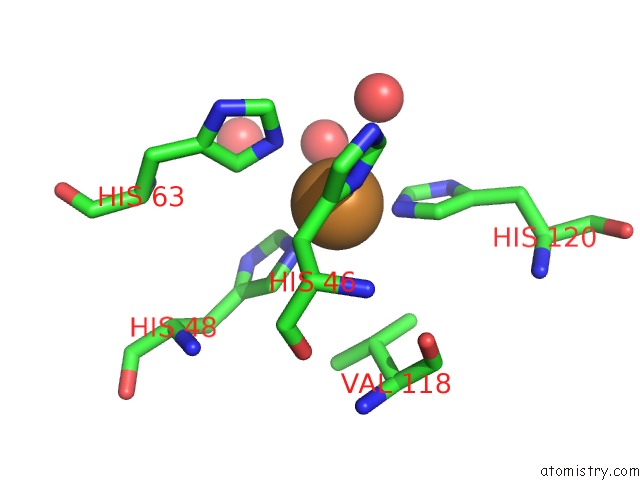
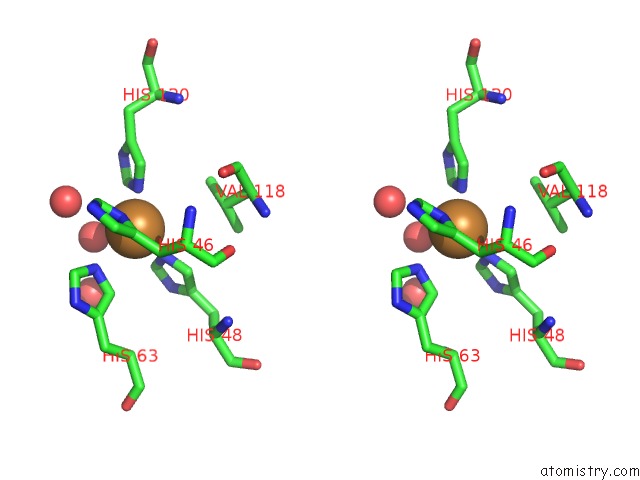
Copper binding site 9 out of 18 in 1hl5
Go back to
Copper binding site 9 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 9 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
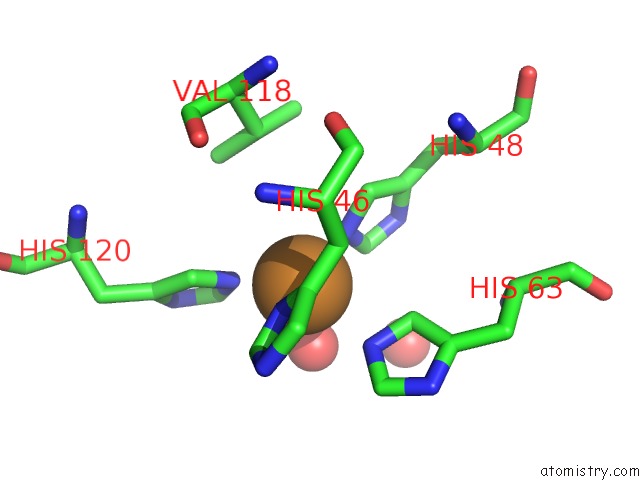
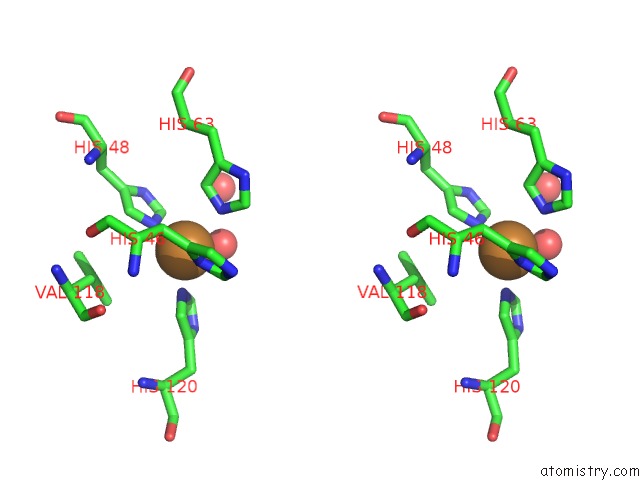
Copper binding site 10 out of 18 in 1hl5
Go back to
Copper binding site 10 out
of 18 in the The Structure of Holo Type Human Cu, Zn Superoxide Dismutase
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Copper with other atoms in the Cu binding
site number 10 of The Structure of Holo Type Human Cu, Zn Superoxide Dismutase within 5.0Å range:
|
Reference:
R.W.Strange,
S.Antonyuk,
M.A.Hough,
P.Doucette,
J.Rodriguez,
P.J.Hart,
L.J.Hayward,
J.S.Valentine,
S.S.Hasnain.
The Structure of Holo and Metal-Deficient Wild-Type Human Cu, Zn Superoxide Dismutase and Its Relevance to Familial Amyotrophic Lateral Sclerosis J.Mol.Biol. V. 328 877 2003.
ISSN: ISSN 0022-2836
PubMed: 12729761
DOI: 10.1016/S0022-2836(03)00355-3
Page generated: Tue Jul 30 21:56:54 2024
ISSN: ISSN 0022-2836
PubMed: 12729761
DOI: 10.1016/S0022-2836(03)00355-3
Last articles
Cl in 7ZWYCl in 7ZX4
Cl in 7ZXD
Cl in 7ZX2
Cl in 7ZWX
Cl in 7ZWT
Cl in 7ZWU
Cl in 7ZW2
Cl in 7ZWS
Cl in 7ZWO